Biology Reference
In-Depth Information
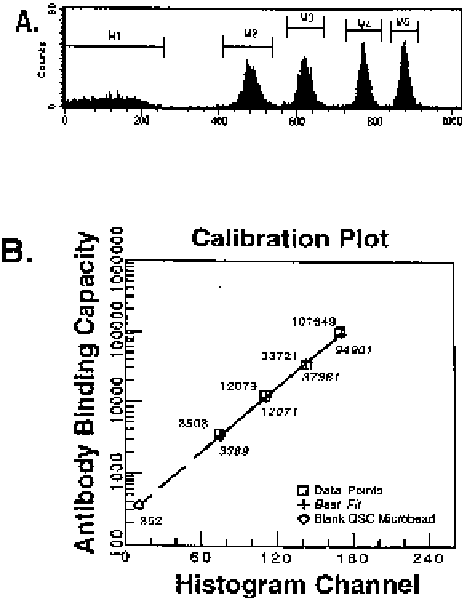
F i g u r e 4.1. Quantitative ¯ow cytometry (QFACS) techniques have been developed that use
populations of highly uniform microbeads that bind speci®c amounts of ¯uorochrome-conjugated
murine immunoglobulins (M1, M2, etc. peaks in A). The beads, approximately the size of human
peripheral blood lymphocytes, are processed and analyzed in the same way as the experimental
samples. A standard curve is generated using the peak channel ¯uorescence intensities of each bead
population (staining of beads with anti-CD4-PE is shown in A, the resulting calibration curve is
shown in B). Thus, it is possible to determine the number of antibody-binding sites on a particular
cell population.
conversion can be done directly, by comparison to a standard curve generated
by a series of microbeads predetermined to bind a ®xed number of antibody
molecules, or indirectly, by comparison to a standard curve generated by beads
conjugated with di¨ering levels of ¯uorochrome molecules. An example of the
former is the Quantum Simply Cellular
TM
(QSC) kit from Sigma (St. Louis,
MO), whereas the QuantiBRITE
TM
(QB) system (Becton Dickinson, San Jose,
CA) is a popular example of the latter system, although many other com-
panies make standardized ¯uorescence beads (e.g., Spherotech, Libertyville, IL;
Molecular Probes, San Diego, CA). Figure 4.1 shows an example of a typical
regression curve generated from the QSC system using a phycoerythrin (PE)-
conjugated anti-CD4 antibody. The system consists of ®ve microbead popula-
tions of uniform size coated with goat-anti-mouse antibodies: four populations
bind an increasing number of mouse antibodies whereas one population does