Information Technology Reference
In-Depth Information
B
C
A
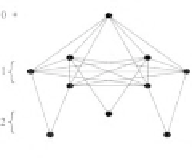
Figure 5.
Three dimensional representation of the oxidative folding space of
polypeptides with 4,5 and 6 cysteine residues (A, B and C, respectively). The nodes
represent intermediates, the number of disulfide bridges is indicated with numbers
on the left of each panel. The edges indicate disulfide exchange transitions. Zero
indicates the fully reduced state, nodes in the lowest plane are the fully oxidized
intermediates, one of which is the native state. Edges within the same plane indicate
shuffling reactions (interchange between two protein-bound disulfides), edges
between planes are redox transitions in which a disulfide bridge is created or
abolished.
The network representations shown in Figure 3 are three-dimensional representation
of the entire oxidative folding space described in terms of chemically well-defined disulfide
intermediates. Species with the same number of disulfide bridges are placed on the same
plane, so shuffling reactions, which do not change the number of disulfide bridges are
represented as edges within the same plane. On the contrary, reactions in which a disulfide
bridge is gained or lost, are represented as edges between two neighbouring planes. The
fully reduced state (zero disulfide bridges) is on top, the fully oxidized species, on of which
is the native state, is on the bottom. Panel B shows a peptide with 5 cysteines, such as
granulocyte-colony stimulating-factor [21, 22] in which the native state contains one free
cysteine residue that is not part of a disulfide bridge. In this case the native state can in
principle rearrange into other species, so there are shuffling edges also in the lowest plane
in the figure. In most of the known cases, the number of cysteines is an even number, so
the fully oxidized DISs cannot readily interconvert into each other. In some cases this
might be an obstacle: the propeptide of BPTI contains an additional free cysteine that seems
to facilitate the folding of the molecule. The propeptide is subsequently cleaved and in this
way the structure is locked into the native disulfide configuration [23]. The oxidative
folding pathways can be pictured as routes within the full network, starting at the fully
reduced species and ending at the native state. In the literature there are a few well-studied
examples in which folding intermediates have been determined. Three examples, bovine
pancreatic trypsin inhibitor, insulin-like growth factor and epidermal growth factor are
shown in Table 2 and Figure 6.
BPTI's folding pathway was the subject of an intense dispute in the early 1990's, but
later resulted in one of the most extensively studied oxidative folding pathways and a major
protein folding model. With some differences, BPTI's pathway was characterised with the
predominance of only a limited number of folding intermediates that adopt mainly native
disulfide bridges and native-like structures. It is important to remember that 1- and 2-
disulfide intermediates were present, but no 3-disulfide species apart from the native
protein was detected on this pathway. One of the most abundant intermediates is a two
disulfide species with two native disulfide bonds and a native-like structure. Formation of
the third disulfide (Cys14-Cys38) is the last step of the folding process. A prevalence of the
native-like structures and native disulfide bridges points to the conclusion that non-covalent