Information Technology Reference
In-Depth Information
favoured over the nucleation model, because they imply the existence of folding
intermediates, which were discovered soon after. All proposed mechanisms and models
were able to explain particular pieces of experimental data, but none provided a clear
explanation of the folding principles or a solution to Levinthal's paradox (for a collection of
reviews see: [3]).
The current, unified view of protein folding presented in some highly cited reviews
by Dobson and co-workers [4,5], underlies the fact that protein folding is a progression in
which both native and non-native contacts stabilise native-like structural features. The
folding either proceeds through a hydrophobic collapse to a compact globule that has
stabilising interactions or through a slow formation of a folding core (nucleus), which then
rapidly proceeds towards the native state. Folding is thus seen as a step-wise behaviour,
sampling regions of the landscape that are downhill in energy. An important element in the
“new view” of protein folding is the folding funnel, which was first introduced by Onuchic
and associates [6]. This is one way of representing the folding landscape with the free
energy (enthalpy and entropy) as a function of folding progress variable, also known as the
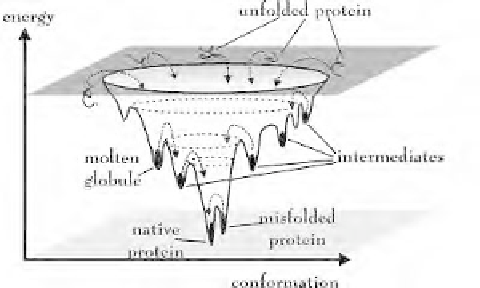
fraction of the native contacts. In the light of this simple surface (see Figure 1), it is
possible to understand a number of features of the folding process. There are three kinds of
states that can be easily distinguished in the folding funnel.
Figure 1.
Schematic representation of the energy landscape of protein folding. The
energy of a protein is displayed as a function of the topological arrangement of
atoms. Adapted from Cemazar [7].
The initial state from which the folding proceeds is extremely heterogeneous and
encompasses a large conformational space of rapidly inter-converting states. It seems
generally accepted that the unfolded or denatured states are not completely random as one
would expect for a theoretical polymer. On the contrary, it has intrinsic propensities for
native and non-native like interactions, which funnel the folding process either through
global or local conformational preferences. Compact denatured states, commonly known as
molten globules, are lower in energy in the folding funnel. These have been in the past
defined with a set of well-defined features such as a set of secondary structural elements in
the absence of tertiary structure. In contrast, at the bottom of the funnel we find a highly
compact state, where the close packing of the side chains is essential for a well-defined
conformation. This is the so-called native state [4,5].