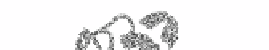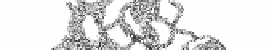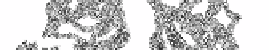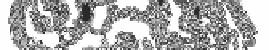Information Technology Reference
In-Depth Information
6.1 Other Soluble Domains
Other alpha-helical domains include the alpha-alpha barrel, here illustrated by the catalytic
core of glycosyltransferase from Clostridium thermocellum shown in figure 26. The barrel
is formed by six inner and six outer alpha helices.
Other common
-
propellers comprise 4 to 8 small anti-parallel sheets with identical up-down topology
arranged like the blades of a propeller. The example below (figure 28) is the head domain
of influenza neuraminidase, which is a six-bladed propeller. As discussed previously,
parallel
β
-structures include the
β
-propellors,
β
prisms and
β
-helices.
β
β
-sheets are usually formed from repeated
βαβ
motifs. However pectate lyase and
the tailspike protein from P22 phage are
-sheets that
form a
β
-helix, classified as a 3-solenoid in the CATH database. The repeat unit that forms
one turn of the helix contains three strands and three loops [91]. From figure 27 it can be
seen that the parallel
β
-structures comprising three parallel
β
-sheets are almost planar with two packed adjacent to each other
while the third sheet is almost perpendicular to the other two. There are related structures
with only two sheets packed together found in bacterial extracellular proteases.
β
Figure 28. a) Neuraminidase from influenza A virus 1f8d, chain A, residues 82-486
[93]. There are 6 anti-parallel β-sheets each shaded differently, forming the 6 blades
of a propeller structure.
CATH:
2.120.10.10 (beta, 6-propeller, neuraminidase)
SCOP
: beta, 6-bladed beta-propeller. b) Topology of neuraminidase (loops not to
scale)