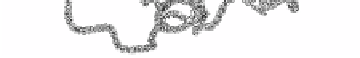Information Technology Reference
In-Depth Information
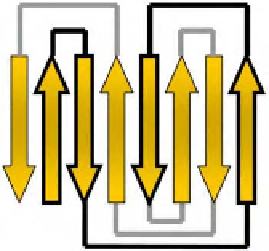
from strand 3 to strand 4 crosses the other way (figure 23 b). Proteins with this fold include
the satellite panicum mosaic virus coat and PAH monooxygenase [81-82].
Immunoglobulin fold.The immunoglobulin constant domain consists of a four-
stranded
-
sandwich with the topography shown in figure 24b. Like the jellyroll, a Greek-key motif is
divided between the layers. Examples of proteins with this fold include Bence-Jones
protein (figure25) and superoxide dismutase [83-84].
Trefoil. The trefoil fold is formed from six two-stranded hairpins, three of which
form a three-sided barrel while the others form a triangular array that caps the barrel, giving
the fold pseudo three-fold symmetry [85]. The fold is found in several protein families
including Kunitz soybean trypsin inhibitors (STIs), ricin-like toxins, plant agglutinins and
hisactophilin-like actin-bundling proteins [85-87].
β
-sheet packed against a three-stranded
β
-sheet to form an aligned 2-layered
β
Figure 22. Major cold shock protein 7.4, 1mjc [88]. Example of an OB Roll. (Side
and end view)
CATH
: 2,40,50,240 (beta, barrel, OB-fold).
SCOP
: beta, OB-fold.
a) b)
Figure 23. a) C-reactive protein, 1b09 [89]. Example of a jellyroll.
CATH
:
2,60,120,200 (beta, sandwich, jellyroll).
SCOP
: beta, concanavalin A-like
lectins/glucanases. b) Topology diagram of a jellyroll. One
β
4
Greek key motif is
outlined in heavy type.