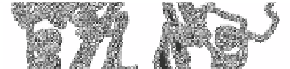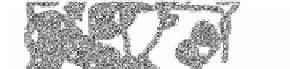Information Technology Reference
In-Depth Information
5.2 Alpha / Beta Domains
αβ
motifs. The
geometry and energetics of
αβ
-packing has been extensively studied [63-64]. The
α
-helix
has 3.6 residues per turn, therefore the
-Packing The super-folds in this class are made up of repeated
βαβ
α
-helix face has a right-handed twist which
complements the right handed twist of the
-sheet when the structures are parallel. This is
the most favoured configuration although another common arrangement has the helix
diagonal to the sheet with interactions between the centre of the helix and centre of the
sheet or the ends of the helix and corners of the sheet depending on whether the helix is
above or below the
β
-sheet. The helix can also be perpendicular to the sheet in which case
contacts can form along the length of the helix. [64-36]. The
β
-sheet contact surfaces
usually comprise small hydrophobic residues such as valine, leucine and isoleucine, which
allow for good close packing [63].
TIM Barrel The TIM barrel (named after triose phosphate isomerase (figure 17))
has a core of eight twisted, parallel
β
-strands that form the 'staves' of a barrel surrounded
by the connecting
β
-strands comprise alternate branched and bulky
hydrophobic residues, the former pack against the helices, the latter create a tightly packed
hydrophobic core. A large proportion of proteins with this structure are enzymes for
example, aldolase and tryptophan synthase [65-66].
α
-helices. The parallel
β
a) b)
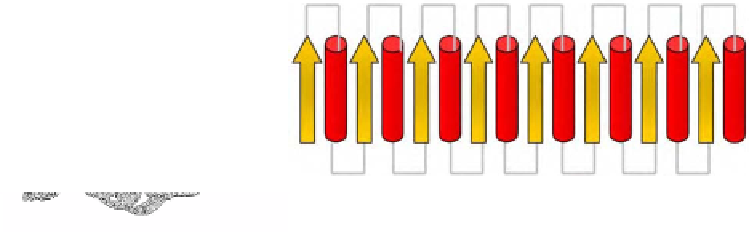
Figure 17. a) Triosephosphate isomerase, chain B, 7tim [70].
CATH
: 3,20,20,90
(alpha beta, barrel, TIM barrel)
SCOP
: a/b, TIM beta/alpha-barrel. b) Topology
(arrow = β-strand, cylinder = α-helix)
Doubly-wound Alpha/Beta Fold (Rossman Fold) Unlike barrels these structures are
open with a central
β
-sheet flanked by helices to form a 3-layered sandwich. The chain
starts in the middle of the
-sheet and travels to the edge, then returns to the centre via a
loop or helix and travels outwards to the opposite edge. Proteins with this structure include
flavodoxin and adenylate kinase [67-68]. The
β
configuration is named the Rossman
fold after Michael Rossman who first described this configuration in nucleotide-binding
proteins [69].
βαβαβ