Information Technology Reference
In-Depth Information
Figure 8. Section of glutathione
reductase, 1dnc, [18] Example of a
planar β-sheet.
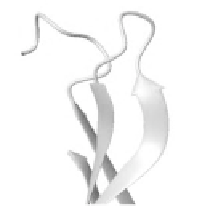
Figure 9. Section of carboxy
peptidase A, 1m4l, [19] showing a
right-handed twisted β- sheet
Loops of less than seven residues form predictable structures known as reverse or
tight turns, which have been categorised according to the number of residues involved. In
each case the turn is not part of a helical structure, the distance between the first and last
residue is less than 7Å and there is usually a hydrogen bond between the first and last
residues in the turn [20].
The smallest is the
turn, which contains two amino acids with a hydrogen bond
between the backbone NH (i) and the backbone CO (i+1) while slightly larger is the
γ
turn
involving three residues with a hydrogen bond between the backbone CO(i) and backbone
NH(i+2).
δ
-Turns have been classified into two types, classic and inverse, based on the
dihedral values of the (i+1) residue [20].
The most abundant reverse turn, which is found in most topological environments,
is the four-residue
β
-turn. Beta-turns were identified by Venkatachalam [21] who used
model building techniques to characterise three favourable conformations in which a
hydrogen bond could form between the backbone CO(i) and the backbone NH(i+3). These
were designated types I, II and III and their more sterically constrained mirror image
conformations were designated types I', II' and III'. Types I and II are identical except that
the second residue is rotated by 180
o
(figure 10) and type III is equivalent to one turn of a
3
10
helix. As the number of known protein structures increased it became apparent that 25%
of
γ
-turns were not stabilised by a hydrogen bond [22] and the definition was broadened
accordingly. In a more recent classification, nine different types of
β
-turn were identified
based on the
β
angles of the second and third residues in the turn (Figure 11) [23-25].
These are designated Types I, II, VII, I', II' VIa1, VIa2 VIb and IV. This scheme is used by
the authors of the program, PROMOTIF [26], which provides details of protein secondary
structure and motifs in the Protein Data Bank and can be accessed at
http://www.biochem.ucl.ac.uk bsm/pdbsum/
φ
and
ϕ