Information Technology Reference
In-Depth Information
3.6
residues
a) b)
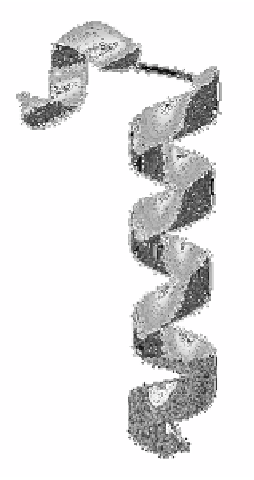
Figure 5 a) RASWIN cartoon of a α-Helix from horse heart myoglobin (1ymb),
[12]. The kink is caused by a proline residue 37 (dark grey) b) RASWIN ball and
stick model of an
α
-helix showing internal hydrogen bonds.
Polyproline II helix Trans poly-L-proline forms a left-handed polyproline II (PPII)
helix with
angles of -75
o
and 145
o
and n=-3.0 [10]
.
In globular proteins short
stretches of PPII helix are found on the protein surface. They tend to be mobile generally
having few main chain hydrogen bonds with the rest of the protein and are stabilised by
hydrogen bonding with the solvent [11]. Proline residues are usually present in the
sequence but this is not obligatory.
φ
and
ϕ
2.2 Beta-Pleated Sheets
Pauling and Cory [13] also predicted the second major secondary structural element in
proteins, the
-strand. Beta-strands have an extended conformation but are technically
helices with two residues per turn so that consecutive residues are rotated by 180
o
with
β
φ
and
ϕ
angles in the upper left-hand allowed region of the Ramachandran map (figures 3 and
4). Strands tend to be 5 to 10 residues long and are aligned with an adjacent strand so that
hydrogen bonding can occur between the C
O of one strand and the NH of the other to form
a sheet structure in which all possible main chain hydrogen bonds are formed. Although
′
-
sheets may involve
β
-strands that are not consecutive in the sequence and are therefore
considered to be tertiary structure by some authors (for example Prztycka and co-workers
[[14]]) it is usually convenient to classify them as secondary structure. Successive C
α
atoms
lie above and below the plane of the sheet so that the structure is pleated. Strands can run
parallel (in the same biochemical direction) or anti-parallel, each form having a distinctive
hydrogen-bonding pattern (figures 6, 7). Sheets can be mixed, parallel and anti parallel but
there is some energetic bias against mixed sheets. Anti-parallel chains can pack more
closely than parallel sheets resulting in shorter interchain hydrogen bonds; furthermore
anti-parallel sheets have well-aligned hydrogen bond dipoles whereas those of parallel
β