Geoscience Reference
In-Depth Information
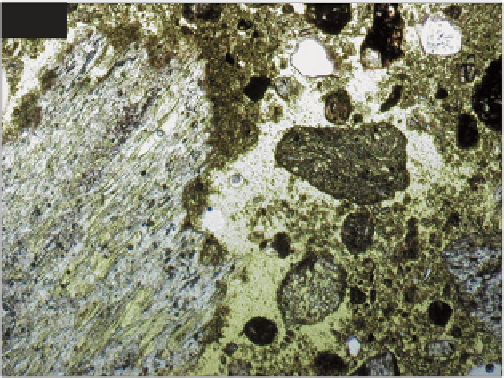
and isotropic hydrated calcium silicate gel (C-S-H), which
is also referred to as 'tobermornite gel'. Within this are
embedded crystallites of portlandite (calcium hydroxide,
approximately 10%) and residual unhydrated cement
grains. In thin section, portlandite (Ca(OH)
2
) is visible in
the cement matrix as anhedral or euhedral crystallites,
plates or short prisms, up to 100 μm in size. They are
colourless in plane-polarized light and in cross-polarized
light they are highly birefringent and stand out against
almost isotropic uncarbonated gel. Over time (years to
decades) the cement matrix gradually reacts with the
atmosphere causing carbonation which alters the matrix
to crystalline calcium carbonate (see p. 96).
When describing the hardened cement matrix of
concrete samples the petrographer would normally
include the following. In hand specimen describe the
colour, distribution, relative hardness, and any evidence
of distress or deterioration Microscopically, the features
described should include the cement type, the nature of
relict cement grains, and the nature of portlandite
crystallites. Description of the relict cement grains and
portlandite crystallites should include size, relative
abundance, distribution, and shape. Size and relative
abundance can be described using the following
classifications:
• Size (average measurement across the grains or
crystallites):
• Small - <20 μm.
• Medium - 20-60 μm.
• Large - 60-100 μm.
• Very large - >100 μm.
• Relative abundance:
• Rare - only found by thorough searching.
• Sporadic - only occasionally observed during
normal examination.
• Common - easily observed during normal
examination.
• Frequent - easily observed with minimal
examination.
• Abundant - immediately apparent to initial
examination.
cement in concrete to be replaced by one or more mineral
additions. When combined with mineral additions,
cements are referred to as 'composite' or 'blended'
cements, in Europe and the USA respectively. Cost savings
gained from replacing cement clinker with industrial waste
products was the original reason for the development of
blended cements. Now mineral additions are used to
confer a range of performance benefits to the concrete mix
and to reduce the carbon footprint of concrete binders.
Mineral additions include a range of natural and
industrially manufactured materials that often exhibit
pozzolanic behaviour. The original pozzolana was volcanic
pumice from Pozzouli, Italy (
173
) that was used by the
Romans in lime concrete/mortar (see also p. 148). The
Romans discovered that when added to lime, the pozzolana
would allow it to set underwater, thus enabling marine
construction. This was due to the pozzolanic behaviour,
which involves a reaction between amorphous silica in the
pozzolana and calcium hydroxide in the lime binder or
cement clinker to form C-S-H. For certain pozzolanas, the
pozzolanic reaction is slower than Portland cement
hydration, with correspondingly lower rates of heat
liberation and strength gain. For Portland cement concrete,
this has the advantage of reducing the risk of early thermal
cracking but with the disadvantage of increasing the curing
time. The binder resulting from use of mineral additions is
often more durable as the pozzolanic reaction confers
reduced permeability (to aggressive agents) and a lower
content of calcium hydroxide (that could otherwise have
participated in deleterious reactions).
In modern concrete, the most commonly used
173
A
DDITIONS AND ADMIXTURES
Additions are inert, pozzolanic, or latent hydraulic
mineral powders, which are added at the mix.
Admixtures are chemicals added to concrete at the time
of mixing. These should not be confused with additives,
which are chemicals preblended with the cement or dry
cementitious mix. Other materials added to concrete
include fibres which are most commonly composed of
steel or plastic.
It is now common practice for a proportion of the
173
Natural pumice pozzolana from Bacoli, near
Pozzuoli (Italy); PPT, ×75.