Geoscience Reference
In-Depth Information
shows a fine aggregate that is contaminated with organic
material. Other forms of organic matter, such as coal and
lignite (see
217
), are undesirable in concrete aggregate as
they are weak, unsound, and can cause staining at
concrete surfaces. ASTM C33 limits the amount of coal
and lignite in concrete aggregate to 0.5% for concrete
elements with visible surfaces and 1% for hidden concrete.
Alkalis
- high levels of alkali metal ions (sodium and
potassium) can cause alkali-aggregate reaction (AAR) in
concrete (in the presence of reactive aggregate and
water). Salt-contaminated aggregates (marine aggregate)
can contain alkalis that can potentially contribute to
ASR. A number of common rock-forming minerals
(feldspars, phyllosilicates) contain appreciable alkalis and
in certain rare cases these have been released in concrete,
causing ASR (Goguel & Milestone, 2007). AAR is
discussed further in the following section and on
pp.109-114.
Sulfides and sulfates
- iron pyrite (iron sulfide) occurs
in some natural aggregate deposits such as the flint
gravels of southeast England. Some pyrites oxidize when
incorporated in concrete aggregate and may cause 'pop-
outs' and brown staining on concrete surfaces (see
216
).
Reactive pyritic aggregate particles can be distinguished
from unreactive ones by immersing them in a limewater
solution. Reactive pyrite particles will form a blue-green
gelatinous precipitate within 5 minutes that changes to a
brown colour within 30 minutes (Midgley, 1958). Sulfate
minerals are present in some aggregate resources and
when incorporated into concrete may cause sulfate
attack. In areas of the Middle East (French & Crammond,
1980) and in tropical coastal plains, sulfates commonly
occur in aggregate resources (
142
).
AGGREGATE FOR CONCRETE
AAR is the expansive reaction between alkali (sodium
and potassium) hydroxides in the pore solution of
concrete and minerals in the aggregates. These are
deleterious reactions that cause expansive cracking of
concrete structures, which detrimentally affects their
durability.
Petrographic examination is the ideal starting point
for the assessment of aggregates for alkali-aggregate
reactivity (Sims
et al
., 2002). In the United Kingdom,
concrete aggregate combinations are classified as being
of 'low', 'normal', or 'high' reactivity on the basis of their
petrographic composition. An aggregate or aggregate
combination is classified as being of low reactivity if it
comprises 97% or more of rock and mineral constituents
considered to be of low reactivity potential. An aggregate
or aggregate combination is classified as being of high
reactivity if it comprises either more than 10% crushed
greywacke or recycled demolition waste. An aggregate is
classified as being of normal reactivity if it cannot be
classified as being of low or high reactivity. Any
aggregate containing detectable opal or opaline silica
should not be classified and, in most circumstances,
should not be used. A list of aggregate and mineral types
142
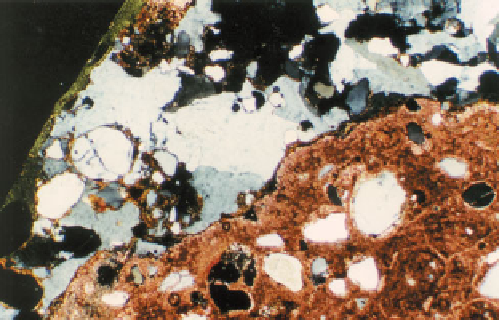
142
Limestone coarse aggregate particle (Saudi
Arabia) including the sulfate mineral gypsum (grey).
The thin section has been stained in accordance with
Dickson's method and the nonferroan calcite of the
limestone appears pink; XPT, ×35.