Geoscience Reference
In-Depth Information
comparison of the ingredients with a reference sample of
the specified plaster. In addition, samples can be screened
for impurities that can have a detrimental effect, such as
loam and clay which affect the setting time and final
strength.
A major drawback of gypsum binders is that they are
water soluble and are therefore only suitable for internal
work. The absorption of just 1% water can reduce strength
by up to half and long-term exposure breaks down the
interlocking structure of the gypsum crystals. They were
sometimes used externally on historic buildings as mortars
and renders, where they usually show signs of severe
weathering. Microscopical investigation of historic
gypsum mortar weathering typically shows the gypsum
binder dissolved away, with the remainder appearing
reprecipitated as larger crystals with a denser
microstructure (Middendorf, 2001). Observation using SEM
is required to study the morphology of the very fine
crystals found in gypsum plasters.
Workmanship issues that can be addressed include
checking that the plaster has been applied using the
specified number of layers, at the recommended
thickness. Finishing plaster can debond if the undercoat
has not had time to set, if it has an inadequate
mechanical key to the undercoat, or if a strong finishing
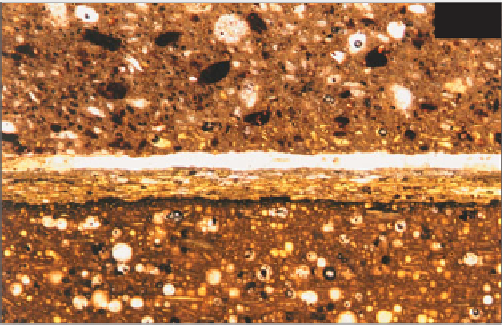
coat is applied to a weak undercoat. Figure
278
shows a
finishing plaster that has debonded from its plasterboard
background. Gypsum plasters are nonshrinking but may
suffer cracking for other reasons when subjected to
movement in excess of their strain capacity. Common
causes of cracking include background shrinkage (if the
background was very wet), base coat shrinkage (with
cement-based undercoats), cracks reflecting plasterboard
joints, and building movement. An unsatisfactory soft
powdery surface may result if the plaster dries out before
it has fully hydrated. This is more likely to happen if thin
coats are applied to a dry background, such as
plasterboard.
I
NTRODUCTION
Lime is formed by burning a source of calcium carbonate
(CaCO
3
), usually limestone or magnesian limestone,
between 850°C and 1200°C, driving off carbon dioxide
to form calcium oxide (CaO, quicklime). The calcium
oxide is then slaked with water (evolving heat) to form
calcium hydroxide (lime, Ca(OH)
2
). The slaking process
can produce dry lime hydrate powders or, if excess water
is used, lime putty. Lime mortars, plasters, and renders
are manufactured by mixing aggregate with a lime
product, either quicklime, lime hydrate powder, or lime
putty, adding water if required. Lime made from pure
limestone (nonhydraulic lime) sets by drying out and
then hardens wholly by absorption and reaction with
carbon dioxide, slowly to become calcium carbonate
again.
Burning
CaCO
3(s)
+ heat
→
CaO
(s)
+ CO
2(g)
Slaking
CaO
(s)
+ H
2
O
(l)
→
Ca(OH)
2(s)
Hardening
Ca(OH)
2(s)
+ CO
2(g)
→
CaCO
3(s)
+ H
2
O
(l)
Lime made from limestone containing siliceous
impurities has a degree of cementitious properties,
allowing it to set underwater (hydraulic lime). This occurs
as calcium and silica from within the impure limestone
react to form materials similar to those found in Portland
cement, such as dicalcium silicate, aluminate, and ferrite
phases, when the rock is calcined. The mechanism of
hardening of hydraulic limes is a combination of
carbonation and hydration of dicalcium silicate.
The principal applications of petrographic examination
to investigation of modern and historic lime mortars are:
• Identifying the type and source of aggregate.
• Identifying the type, origin, and manufacturing
process of the binder.
• Direct estimation of mix proportions.
• Enabling correction of mix proportions determined
by simple chemical analysis.
• Identifying and quantifying the presence of
inclusions, additives, and impurities.
• Assessing workmanship.
278
278
Natural gypsum finishing plaster (upper)
debonded from paper-faced plasterboard (lower). The
plasterboard comprises gypsum (brown) with
abundant entrained air voids (white, spherical). The
heavy paper facing is clearly visible running
horizontally across the centre of view; PPT, ×35.