Geoscience Reference
In-Depth Information
steam curing (precast units), large pours (mass concrete or
sections >0.5 m in size) or high ambient temperatures (in
tropical climates). The use of certain mineral additions such
as GGBS or PFA helps to reduce the peak curing
temperature and prevent DEF. In reinforced concrete
suffering from DEF, the outer surface will often exhibit map
cracking while subparallel cracks form at depth. In
unreinforced concrete, large cracks (up to 20 mm in width)
may form both subperpendicular and subparallel to the
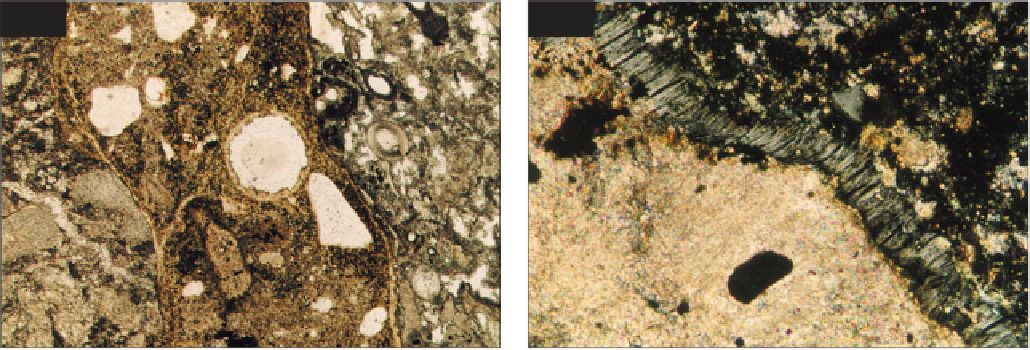
outer surfaces. In thin section, concrete suffering from DEF
exhibits distinctive partings around aggregate particles and
microcracks in the cement paste that may both be filled
with ettringite (Eden
et al
., 2007). Figures
210
and
211
show the appearance of concrete from a large
in situ
concrete pour suffering from DEF. DEF is diagnosed by the
presence of partings around aggregate particles (that
usually become wider as particle size increases), the
absence of an external source of sulfate, and a high
temperature curing history. DEF may occur in combination
with other deleterious reactions such as ASR, in which case
it may be difficult to determine what the dominant
mechanism of deterioration is (Thomas
et al
., 2007).
concrete is reinforced, chloride-induced corrosion of steel
reinforcement. The relative significance of each form of
attack will depend on the exposure conditions of the
structure, with the intertidal and splash zones usually
being most at risk. Only chemical attack by sea water
will be considered here as the other mechanisms are
discussed in other sections.
Open sea water has a pH of 7.5-8.4 and contains 3.1-
3.8% soluble salts (by weight) consisting (approximately)
of 2% chloride, 1.1% sodium, 0.28% sulfate, 0.14%
magnesium, 0.05% calcium, and 0.04% potassium. In
seas that are partially enclosed, such as the Arabian Gulf,
salt concentrations can rise considerably (5-10%).
Chemical attack of concrete is mainly due to the presence
in sea water of magnesium sulfate (Lea, 1970), which acts
on the cement matrix. Magnesium sulfate reacts with the
free calcium hydroxide (portlandite) to form calcium
sulfate (gypsum) and precipitating magnesium hydroxide
(brucite) as follows:
Ca(OH)
2
+ MgSO
4
.7H
2
O
CaSO
4
.2H
2
O + Mg(OH)
2
+
5H
2
O
→
The magnesium sulfate also reacts with the calcium
silicate hydrates of the cement matrix to form more
gypsum, brucite, and hydrated silica as follows:
3CaO.2SiO
2
.nH
2
O + 3MgSO
4
.7H
2
O
S
EA WATER ATTACK
Concrete in marine environments is subject to
deterioration from chemical attack, abrasion, freeze-thaw
mechanisms, salt crystallization/scaling, and, if the
CaSO
4
.2H
2
O +
→
3Mg(OH)
2
+ 2SiO
2
.nH
2
O
210
211
210
Concrete suffering from DEF showing ettringite-
filled partings around coarse aggregate particles and
ettringite deposits lining a small air void; PPT, ×35.
211
Close view of concrete suffering from DEF
showing an ettringite-filled (grey) parting around a
limestone coarse aggregate particle (pale pink). The
ettringite crystals are aligned perpendicular to the
crack; XPT, ×300.