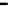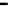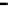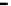Environmental Engineering Reference
In-Depth Information
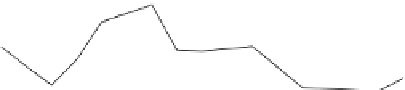
14000
12000
10,470
10000
9,213
7,297
8000
Pan 1 - control
Pan 2 - biostimulation
Pan 3 - bioaugmentation
6000
7,239
4000
2000
933
904
0
0
2
4
6
8
10
12
14
16
18
Time (study months)
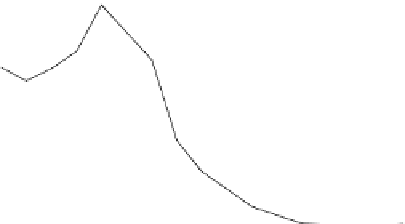
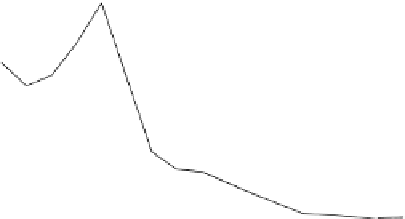
Figure 7.29
A comparison of treatment effects on PAH removal from the troughs.
the three troughs is shown in Figure 7.30, where panel A represents the
control, panel B the biostimulation treatment, and panel C the biostimulated
and bioaugmented scenario.
Using normalized concentrations (Figure 7.31) to take into account the
addition of the bulking material, total PAH reductions of 12, 86, and 87%
were observed in troughs 1, 2, and 3, respectively after 16 months of treat-
ment. An initial rapid decrease in PAH concentration was observed in trough
3, which achieved 50% degradation at 6 months.
Trough 2 followed, achieving the 50% level at 8 months. No significant
reduction was observed in trough 1 after 16 months of treatment. The PAH
degradation during months 4 to 16 in troughs 2 and 3 followed a first-order
decay curve. The trough 2 kinetic coefficient was -5.08e
-1
(r
2
= 1.322), and
the trough 3 kinetic coefficient was 3.464e
-1
(r
2
= 0.616).
The PAH reduction is shown for individual homologues over time in
Table 7.18. Trough 3 is shown as being representative of both trough 2 and
3 reductions. The degradation of PAHs appears to proceed in a pattern
similar to that observed in the LTUs, with lower-molecular-weight homo-
logues being degraded first, followed by consecutively larger molecules.
When a methyl group is attached to a structure, the degradation is delayed,
as in naphthalene and 2-methylnaphthalene. These observations are
supported by the work of Leblond et al. (2001) on PAH structure-activity
relationships. It was also observed that degradation into the next higher ring
number did not require that the lower ring compounds be completely
degraded. Work is in progress to attempt a correlation between structure
and degradation.