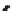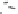Environmental Engineering Reference
In-Depth Information
MSR = 0.20 (30
°
C)
MSR = 0.38 (30°C)
MSR = 0.33 (22°C)
0.35
MSR = 0.14 (22
°
C)
0.35
0.3
0.3
R
2
= 0.993
R
2
= 0.994
0.25
R
2
= 0.998
0.25
R
2
= 0.997
0.2
0.2
0.15
0.15
0.1
0.1
0.05
0.05
0
0
0
0.5
1
0
1
2
3
Tween 80, mM
Tergitol NP-15, mM
Figure 6.22
Solubility enhancements of 4-CBP in solutions of Tween 80 and Tergitol
NP-15 at 22 and 30˚C.
0.14, respectively. These findings indicate that Tween 80 has more than twice
the capacity to solubilize 4-CBP than does Tergitol NP-15. The MSR values
obtained above may be expressed as a micelle-water partition coefficient
(
K
mw
) defined as
X
X
K
=
m
(6.2)
mw
a
where
X
m
is the mole fraction of organic species in the micellar phase and
X
a
is the mole of organic in the aqueous phase. The corresponding log
K
mw
values for 4-CBP in solutions of Tween 80 and Tergitol NP-15 were 6.10 and
5.85, respectively (for calculation details, see Pennell et al., 1997; Laha and
Luthy, 1992). The Tergitol NP-15 value is consistent with existing log
K
ow
-log
K
mw
correlations for alkylphenol ethoxylates, which yielded a log
K
mw
value
of 5.7 (see Pennell et al., 1997).
6.4.5 Mathematical modeling
To predict the effect of surfactant addition on the distribution of PCB con-
geners in a solid-liquid system, it is necessary to account for the potential
impact of sorbed-phase and micellar surfactants on PCB phase distributions.
Surfactant micelles will act to increase the amount of PCB in solution; how-
ever, sorbed-phase surfactant may also increase partitioning of the PCB to
the solid phase. This effect will be a function of the surfactant critical micelle
concentration (CMC), the soil sorption capacity for the surfactant (
S
m
), and
the partitioning of the PCB congener among the aqueous, micellar, and solid
phases. The overall or apparent solubility of a compound in the presence of
surfactant can be represented as the amount of solute associated with