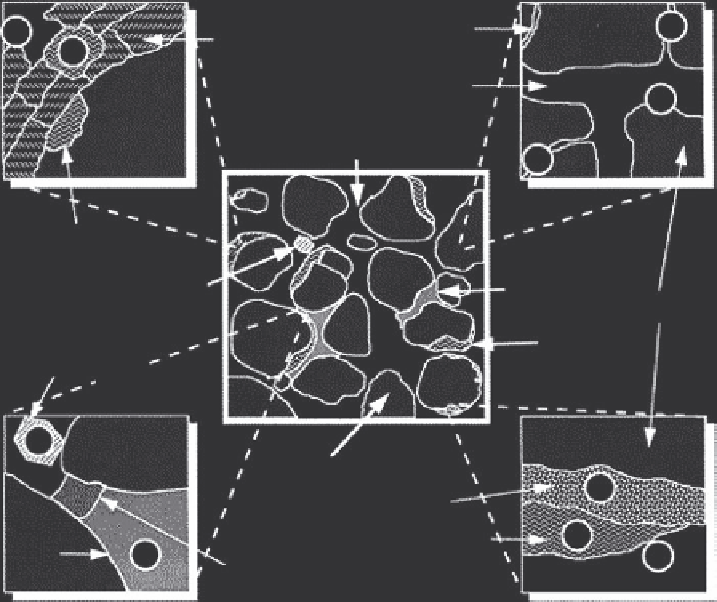
Environmental Engineering Reference
In-Depth Information
E
Micropores
D
Clay Particles
and/or Oxide
Coatings
A
Mesopores
D
Water or Gas
in Macropores
E
Encapsulated
Amorphous SOM
Combustion Residue
NAPL
Mineral Phase
SOM
Combustion
Residue, e.g., Soot
B
Geosorbent
B
Dense SOM
A
Amorphous SOM
Aged or Weathered NAPL
NAPL
A
C
Figure 1.1
Conceptual model of where recalcitrant compounds may reside in soils
and sediments. (Adapted from Luthy, R.G. et al.,
Environ. Sci. Technol.
, 31, 3341-3347,
1997.)
exhibit fast kinetics with low desorption energy and high extractability,
whereas case B would show slow kinetics, high desorption energy, and low
extractability. Case C is characterized by fast kinetics with low desorption
energy and high extractability. Case D would exhibit fast kinetics, low des-
orption energy, and high extractability, with case E showing slow kinetics,
high desorption energy, and low extractability. Assuming all other environ-
mental parameters are consistent among the five different cases, a qualitative
ranking of bioavailability is as follows: BA, BC, BD > BB, BE, where BA, BB,
BC, BD, and BE are the bioavailabilities for cases A, B, C, D, and E.
These different domains within soils/sediments illustrate how structural
and chemical heterogeneity can significantly affect how recalcitrant com-
pounds behave. Although some correlation may be hypothesized with
respect to bioavailability, it is difficult to specify the exact role each sorbent
domain may have. For example, adherent or entrapped anthropogenic
organic matter can also function as a sorbent (e.g., surfactants, soot, or
NAPLs such as oils and tars) (Edwards et al., 1994; Gustafsson et al., 1997;
Boyd and Sun, 1990). Also, there is a growing awareness that the affinity of
nonpolar organics for SOM depends on the SOM's origin and geologic