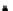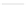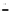Environmental Engineering Reference
In-Depth Information
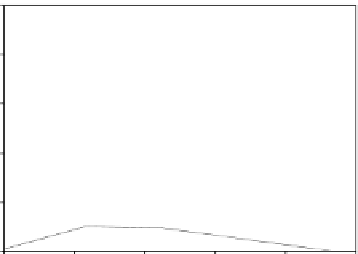
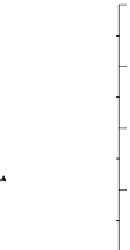
Growth of LB400 (pRO41) on 2CBA
0.5
4.0
OD, 3 mM 2CBA
OD, 2 mM 2CBA
OD, 1 mM 2CBA
2CBA, 3 mM
2CBA, 2 mM
2CBA, 1 mM
Cl
−
, 3 mM 2CBA
Cl
−
, 2 mM 2CBA
Cl
−
, 1 mM 2CB
A
3.5
0.4
3.0
2.5
0.3
2.0
0.2
1.5
1.0
0.1
0.5
0.0
0.0
0 0 0 0 0
100
120
0
Time, hours
Growth of control LB400 (pRT1) on 2CBA
2.50
0.5
2.25
2.00
0.4
OD, 2 mM 2CBA
OD, 1 mM 2CBA
2CBA, 2 mM
2CBA, 1 mM
Time, h vs Col 16
Time,
h
vs Col 17
1.75
1.50
0.3
1.25
0.2
1.00
0.75
0.1
0.50
0.25
0.0
0.00
0
20
40
60
80
100
Time, hours
Figure 6.15
Growth of LB400(pRO41::
ohb
) and LB400 on 2-CBA.
2-CBA. In conjunction with data by Bedard et al. (1986) on co-oxidation of
2-CBA (without growth), it could be that the biphenyl dioxygenase (BDO)
from LB400 does have 2,3-dihydroxylation/dehalogenation activity on both
2-CBA and 3-CBA, unless LB400 possesses another chlorobenzoate-dehalo-
genating enzyme. In this case, no catechol would be produced, in accordance
with the inability of LB400 to grow on 2- and 3-CBA. To verify this, we
PCR-amplified and cloned the BDO genes in
E. coli
, and the
bphA1A2A3A4B
genes appeared to be responsible for the partial dechlorination of 2-CBA.
However, no product of this dechlorination process has been identified.
Strain LB400 is one of only a few organisms known to efficiently oxidize
an environmentally important 2-2′-CB. Haddock et al. (1995) and Arnett et
al. (2000) showed with purified BDO that the initial dihydroxylation of
2,2′-CBP occurs in 2,3 positions with the release of chlorine. Consequently,
the dechlorinated ring is further oxidized to pentadiene, which can be con-
sumed for growth, whereas the second ring converts to 2-CBA.
LB400(pRO41) grew on 1 m
M
2,2′-CBP and released 2.2 m
M
Cl
-
, as