Environmental Engineering Reference
In-Depth Information
~25 Months Post-Flush
~31 Months Post-Flush
0.0125 mg/L
C7
0.0100 mg/L
C7
C3
C4
C3
C4
0.0075 mg/L
MW-512
MW-512
MW-513
MW-513
MW-514
MW-514
C2
MW-505
C2
MW-505
C1
C1
0.0050 mg/L
MW-506
MW-506
MW-509
MW-510
MW-511
MW-509
MW-510
MW-511
0.0025 mg/L
MW-507
MW-507
0.0000 mg/L
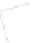
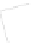
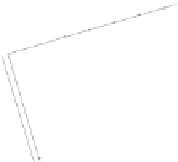
Figure 5.29
Ethylene contour plots over the groundwater monitoring period (12.5
μg/l = 446 μ
M
).
would produce four chloride ions. It is not expected that we see this rela-
tionship in an open system, and these were the highest concentrations mea-
sured over the entire sampling period. The targeted zone does consist of
materials with a relatively low hydraulic conductivity, and the range in
hydraulic gradient at the site suggests that overall movement of groundwa-
ter through the site could be from 15 to 33 m/year.
Partial oxidation of ethanol will result in the formation of 1 mole of acetic
acid and 2 moles of hydrogen gas (Equation 5.2), whereas complete oxidation
of ethanol will result in the formation of 2 moles of carbon dioxide and 6
moles of hydrogen gas (Equation 5.3). Acetic acid (>200 μ
M
) was observed
in all wells except for MW-506, MW-507, MW-511, and C4 (Figure 5.34).
Acetic acid was not measured in MW-506 (upgradient), MW-507 (south of
injection/extraction zone), and MW-511 (farthest well southwest of moni-
tored area), and these same wells never had detectable ethanol
concentrations during the monitoring period. C4 (downgradient and farthest
well northwest of monitored area) had measured concentrations of acetic
acid from 200 to 600 μ
M
after 2 years, even though ethanol was never
detected at this location.
Partial oxidation of ethanol to acetic acid and hydrogen gas:
CH
3
CH
2
OH + H
2
O
CH
3
COOH + 2H
2
(5.2)
Complete oxidation of ethanol to carbon dioxide and hydrogen gas:
CH
3
CH
2
OH + 3H
2
2CO
2
+ 6H
2
(5.3)
Initially, the contour plots of acetic acid show increasing concentrations
in the area of the cosolvent flushing test (Figure 5.35 to Figure 5.37). This
trend continues for approximately 19 months, after which the overall mea-
sured concentrations decrease, and the area of highest concentration is far-
ther downgradient in the area of MW-510 and MW-513. This coincides with
the decrease in ethanol concentrations after 19 months, as would be expected,