Biology Reference
In-Depth Information
HT protein
purification
Quantitative binding
assays
→
structural model
Quantitative binding and
kinetic assays
→
parametrised model
Parameters
Top-down
analyses
Model refinement
Variables
Database and
bioinformatics
Omics measurements
of systems variables
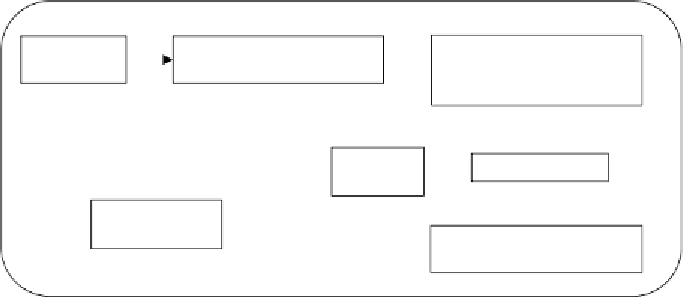
Figure 5
A largely 'bottom-up' strategy for systems biology.
date is the demonstration that the operation of yeast glycolysis under particular
conditions can indeed be rather well predicted on the basis of the 'properties'
of the isolated enzymes which participate in the overall process (Teusink et al.,
2000) (and see (Pritchard & Kell, 2002)). It takes its strongest form when the
interactive properties of all the relevant components of the system are put into a
precise mathematical model, that is a computer replica ('silicon cell', see below)
of the actual system; and if the system behaviour is then calculated successfully.
Occasionally it is argued that such a silicon-cell replica of an actual living
cell would be completely reductionistic and therewith incapable to deal with
the systems biology of the living cell. This is incorrect. Save for vital force
influences, and given an initial physiological condition (cf. below), all there is in
the living cell, at least in one way of looking at it, is a large number of molecules
and all their interactions. Therewith, all that matters is the components and the
relational properties of those molecules. If molecules and interactions (in their
spatial context) are precisely reproduced in a computer program, then all system
behaviour should emerge. The crux resides in the live interaction between the
molecules both in the cell and in the computer program. Here one type of
macromolecule carries out a process for a little while, by which it changes its
environment in terms of a few, nameable properties such as the concentration of
micromolecules like ATP, whilst leaving the rest of its environment unaltered
(see below). The change in environment leads to a change in behaviour of
other types of macromolecules in the same environment in the same cell (e.g.
other enzymes in the same metabolic pathway). The altered behaviour of the
latter molecules will again change the environment of the first macromolecule
and therewith the behaviour of the former. In this way the activity of the first
molecule depends on its own properties through the dynamic activities of the
other molecules. Loosely formulated, it is the resonance with other molecules that