Agriculture Reference
In-Depth Information
up in their higher forms, unavailable for uptake. When the soil becomes acidic to a more
extreme level, e.g. below 4.0, it then becomes possible that certain elements may become
too available, leading to hydrogen, aluminum, or iron toxicity. These can directly damage
root cell membranes and cause other adverse effects.
The Risks of Aluminum Toxicity
“Al [aluminum] damages roots in several ways: In root tips Al interferes with the uptake of Ca
[calcium], an essential nutrient, as well as binds with phosphate and interferes with production of ATP
and DNA, both of which contain phosphate. Al can also restrict cell wall expansion causing roots to
become stunted.” via
Wikipedia
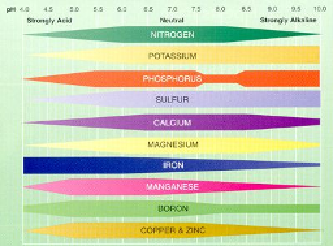
To see the relationship between soil pH and nutrient availability, the following chart shows
how nutrients like nitrogen become less available as the pH drops below 6.0 and others
like trace elements drop off above 6.0. Thus, keeping the pH in balance while applying
electroculture becomes increasingly important, especially over longer periods of time due
to the travelling acid and base fronts.
Nutrient Availability & pH
Source:
Extension.org
It is worth noting that if deleterious long-term electrification effects are observed due
to pH problems, the growing area can be remedied through augmentation of the soil
with buffering compounds such as lime. Amendments can replace the over-abundance
of free hydrogen ions while also supplying additional nutrients to the soil like calcium
and magnesium. Liming also helps with making macro-nutrients like phosphorus more
available. Alternatively or in-conjunction with liming, the reversal of the electric-field
polarity can be helpful as well by causing a reversal of the acid/base fronts.