Agriculture Reference
In-Depth Information
Effect on pH
pH Basics
With all of the electrically-induced ionic and water-based transport occurring within the
soil, one side effect that can have a significant impact is the change to soil pH. For those
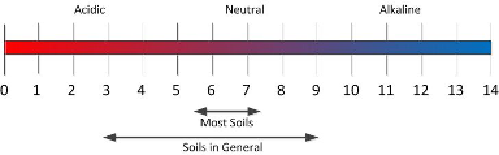
who don't know, soil pH is a measure of the acidity or basicity of soils which affects how
available nutrients are for uptake by the plants. It is expressed as a negative logarithm of
the hydrogen concentration on a scale from 1.0 to 14.0. Values below 7.0 are considered
acidic while those above 7.0 are considered basic or alkaline. A pH of 7.0 is defined as
neutral.
pH Scale
Intermsofthepreferencesofplants,notethatmostplantsprefer(slightly)acidictoneutral
soils, so let's begin by noting how soils become acidic in the first place. While you may
know that pH is a measure of the number of hydrogen ions in a solution, when it comes to
soils, the amount released into the soil is also a function of the number of aluminum (Al
3+
)
ions also present in the soil solution. Aluminum has an interesting property in that it reacts
with water in such a way that causes a 3x release in the number of H+ ions, causing rapid
acidification of soils. Other processes that can make soils more acidic include:
• Fertilizer usage
• Plant root activity
• Weathering of minerals
• Acid rain
pH in Electroculture
Referring to electrolysis reactions shown in Equations 1 and 2, when electric current is
passed throughasolution, the
hydrolysis
,orseparation ofwater,isinitiated. This results in
either oxidation or reduction half-reactions that occur in the electrodes, which results in a
change in pH.