Biology Reference
In-Depth Information
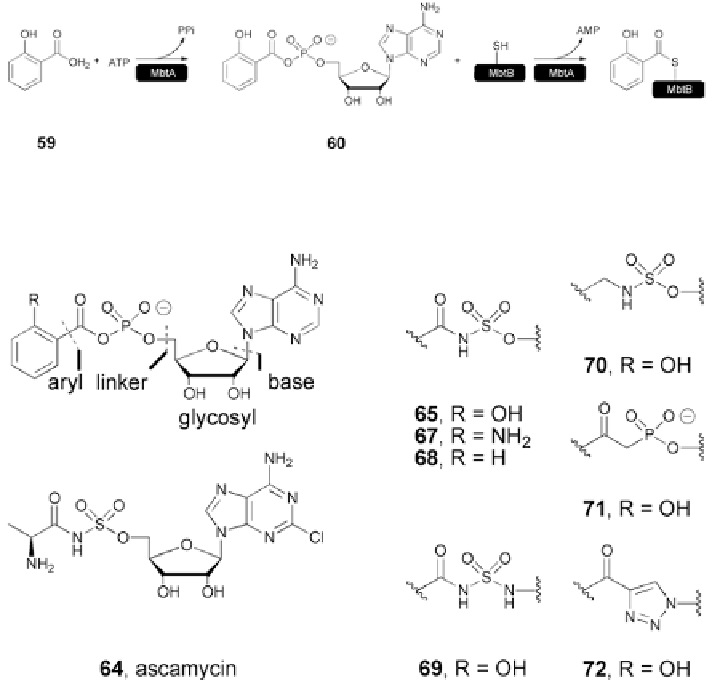
Fig. 5.17
Adenylate-forming reaction catalyzed by MbtA [
73
]
Fig. 5.18
Rationally designed nucleoside antibiotics. SAR of the linker portion [
73
]
Substitution on the salicyl ring demonstrated the importance of the hydroxyl
group. Inhibitor
68
(MIC
99
=
12.5
μ
M) displayed a 66-fold decrease in activity
over analog
65
(MIC
99
=
0.29
μ
M), while the aniline
67
had further diminished
activity (MIC
99
> 100
μ
M). Inhibitors
70
-
72
demonstrated no growth inhibi-
tion (MIC
99
> 100
μ
M). In the case of analog
70
, modeling confirmed the loss
of hydrogen bonding with a lysine residue (Lys519). The completely ionized
phosphate in analog
71
probably limits permeability through the hydrophobic cell
envelope. Compounds
65
and
69
were tested for their ability to block siderophore
synthesis. The addition of
65
(20
μ
M) or
69
(10
μ
M) completely inhibited the
production of both mycobactin T and carboxymycobactin T.
Hydrolysis of the acylsulfamate
65
and acylsulfamide
69
linker present in nucleo-
side antibiotics releases highly cytotoxic adenosine fragments
75
and
76
(Fig.
5.19
).
The possibility of this reaction in vivo would limit the application of the inhibitors.
To address this concern, Vannada [
77
] reported the synthesis of
73
and
74
where
the central nitrogen was removed.
β
-Ketosulfonamide analog
73
displayed modest
Search WWH ::

Custom Search