Biology Reference
In-Depth Information
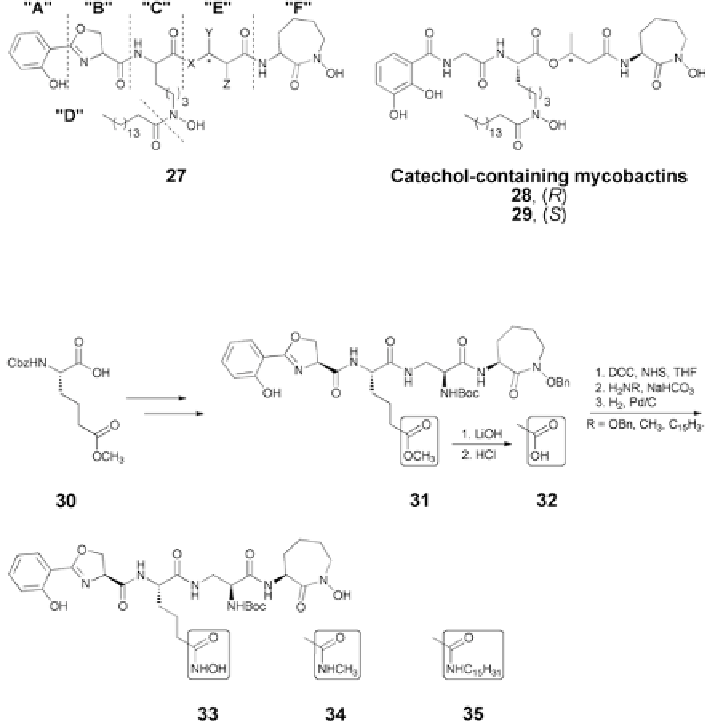
Fig. 5.8
Generalized mycobactin structructure (
27
), catechol-containing analogs [
59
]
Fig. 5.9
Modification on the component “C” of the mycobactin structure [
58
]
Changes of the linear lysine hydroxamate component “C” also were not tol-
erated. Thus, shortening the side chain of
23
to an acetyl as in mycobactin S2
resulted in loss of inhibitory activity against
M. tuberculosis
. Replacement of the
linear
ε
-
N
-hydroxy lysine with an
α
-amino adipate allowed syntheses of a series of
analogs
33
-
35
(Fig.
5.9
) with variation of lipophilicity and iron binding capabili-
ties. Not surprisingly, none of these compounds displayed anti-TB activity.
Since all of the components A-F and related fragment-analogs of the general-
ized mycobactin structure
27
were synthetically available, a number of “truncated”
mycobactins were also synthesized (Fig.
5.10
), tested and found to be inactive
against
M. tuberculosis
[
58
].
In the development of a platform for the convergent manipulation of mycobac-
tin templates that retained the core structure of the natural mycobactins, Juárez-
Hernández et al., reported the syntheses of mycobactin analogs
47
-
49
(Fig.
5.11
)
Search WWH ::

Custom Search