Biology Reference
In-Depth Information
case of Gram-negative bacteria (left panel). The action of a TonB/ExbB/ExbD sys-
tem allows the ferri-siderophore to access the periplasmic space where it is rec-
ognized by a periplasmic binding protein (PBP) that assists in the delivery to the
cytoplasm (4) in an ATP-dependent process (5). Iron release from the siderophore
may occur by metal reduction to its ferrous form, which displays a reduced affinity
with the chelator. Degradation of the ferric-siderophore can also occur in order to
release the metal. Siderophore-acquisition by Gram-positive bacteria occurs is a
comparable fashion (right panel), where the ATP-Binding Cassette (ABC) proteins
involved in the active transport of ferric-siderophores are related to the system
found in Gram-negative bacteria [
21
-
25
].
5.3 Siderophore System in
M
.
tuberculosis
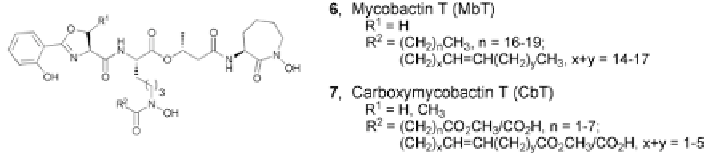
To acquire iron,
M. tuberculosis
secretes two structurally-related siderophores
(Fig.
5.3
). Mycobactin T (
6
) discovered by Snow in 1965, is characterized by the
presence of a long hydrophobic alkyl chain [
29
,
30
]. Initially proposed to func-
tion as a membrane-bound, temporary Fe
3
+
-storage, recent evidence indicates
that Mycobactin T is a more active player in the iron-uptake by
M. tuberculosis
[
31
,
32
]. The second chelator, carboxymycobactin T (
7
), was isolated in 1995 by
Gobin from virulent
M. tuberculosis
ATCC 35801 and avirulent
M. tuberculosis
H
37
Ra. Initially referred to as exochelin, its structure was found to be similar to
that of mycobactin T albeit with a shorter alkyl chain and the presence of an ion-
izable group, rendering a more water-soluble siderophore [
33
,
34
]. Analogous to
other siderophore systems, under iron-sufficient conditions the biosyntheses of
mycobactin T and carboxymycobactin T are regulated at the genetic level by the
ferrous-complex of the repressor protein IdeR [
35
]. A comprehensive review by
Quadri describes the recent findings regarding mycobacterial iron acquisition [
36
].
A clearer idea regarding role of the hydrophobic mycobactin T was observed
when Luo et al. [
32
] studied the effect of mycobactin in iron-uptake within human
macrophages. By monitoring the formation of ferric-siderophore, mycobactin J (
4
)
was demonstrated to be capable of acquiring iron from the macrophage's intra-
cellular pools. The addition of metal-complexes of human transferrin (hTf) influ-
enced the formation of the ferric-siderophore possibly by metal release from the
Fig. 5.3
Siderophores secreted by
M. tuberculosis
[
34
,
61
,
62
]
Search WWH ::

Custom Search