Biology Reference
In-Depth Information
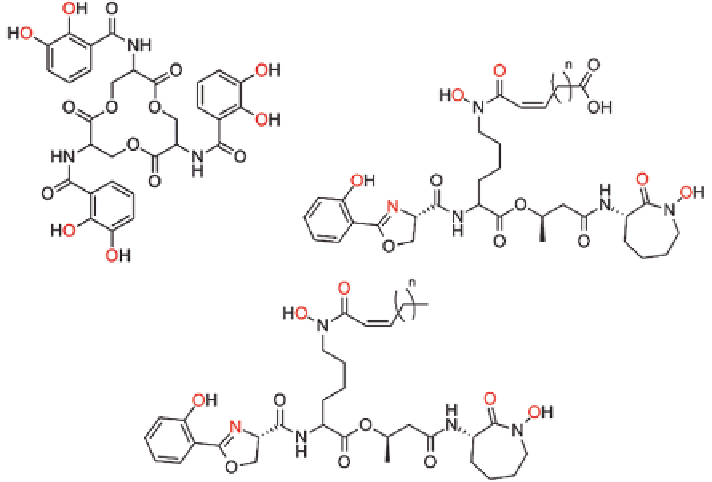
Fig. 4.2
Siderophores enterobactin (
top left
) and generic versions of carboxymycobactins (
top
right
, n
=
3-10) and mycobactins (
bottom
, n
=
6-17). Both carboxymycobactins and mycobac-
tins have varying alkyl substituents on the backbone that, for simplicity, are not included in this
figure
and display compromised survival [
5
,
7
]. The role of Scn as an antimicrobial pro-
tein was further demonstrated by the compatibility of the Scn calyx with a collec-
tion of siderophores from both Gram-positive and Gram-negative bacteria [
8
-
10
].
Recent work has also shown that Scn is a mediator of mammalian iron trans-
port, utilizing so-called mammalian siderophores (simple catechols found endog-
enously in the mammalian gut) [
11
,
12
]. Scn-mediated iron transport is thought to
occur in kidney embryogenesis or in cases of kidney damage where concentrations
of iron must be strictly regulated to control inflammation. Catechols are iron-bind-
ing moieties found in some natural siderophores (e.g., enterobactin, Fig.
4.2
) and
can be bound by Scn as either the free ligand or the iron complex. Endogenous
catechols, found as byproducts of either bacterial or human metabolism, are biden-
tate iron chelators. Under physiological conditions, ferric
bis
catechol complexes
primarily are formed, as determined by speciation calculations [
11
]. Scn intercepts
these complexes and recruits a third catechol to fill the iron coordination shell such
that the ferric complex is hexacoordinate. Ferric
tris
catechol complexes carry a
−
3
charge and the additional aromatic catechol optimizes binding by Scn via hybrid
Coulombic and cation-pi interactions. However, not all catechols are mamma-
lian siderophores. Those with substituents on the aromatic ring exhibit a severely
compromised affinity for Scn. The consequent decrease in affinity is due to steric
clashes between catechol substituents and the rigid Scn calyx [
13
,
14
].
Search WWH ::

Custom Search