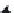Biology Reference
In-Depth Information
integral proteins involves the use of detergents. This large topic is discussed in Chapter 13
describing protein reconstitution into membranes.
Although thousands of globular proteins have been crystallized and their 3-D structures
accurately resolved by crystallography, only a few dozen integral membrane proteins have
been resolved
[12]
. The problem of obtaining 3-D structures for the integral proteins is in
obtaining pure crystals in the first place
[13]
. While peripheral proteins are obtained as mono-
mers that are free of membrane lipids, integral proteins are isolated as aggregates with lipids
(or detergents) still attached. The aggregates are almost impossible to crystallize. Therefore
structural information about integral proteins is often obtained by indirect methods like
enzyme degradation studies and hydropathy plots.
a. Endo and Ecto Proteins
Endo and ecto proteins do not traverse the entire membrane. Instead they are inserted into
the membrane's hydrophobic interior from either the cytoplasmic side (endo protein) or the
extracellular side (ecto protein). An example of such a protein is cytochrome b
5
. Cytochrome
b
5
is an electron transport protein associated with the endoplasmic reticulum (ER). It is
a component of the mixed function oxidase system involved in the desaturation of fatty acids.
Intact cytochrome b
5
can only be removed from the ER membrane by detergents. It is there-
fore by definition an integral protein, but since it has none of its 152 amino acids exposed on
one side of the membrane, it is not a trans-membrane protein. When exposed to trypsin the
cytochrome is cleaved at the aqueous interface producing a 104 amino acid water-soluble
N-terminal peptide and a 48 amino acid membrane soluble C-terminal peptide (
Figure 6.5
)
[14]
. The C-terminal membrane-bound segment has many hydrophobic amino acids and is
depleted in hydrophilic amino acids.
b. Trans-membrane Proteins
A trans-membrane protein must span the entire membrane with substantial segments
exposed on both the outside and inside aqueous spaces. The membrane that must be
trypsin
104 amino acids
152 amino acids
48 amino acids
CYTOCHROME b5
FIGURE 6.5
Cleavage of cytochrome b
5
by trypsin producing a 104 amino acid water-soluble protein and
a membrane-bound 48 amino acid peptide.