Biology Reference
In-Depth Information
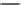
FIGURE 5.3
Hydrolysis of triacyl-
glycerol (saponification) produces
glycerol and 3 K
þ
salts of fatty acids.
O
C
3K
+
R
3
-
O
O
C
R
1
CH2
O
+
O
C
O
C
R
2
CH2
OH
CH
O
KOH
R
3
CH
OH
CH2
O
CH2
OH
glycerol
least since the ancient Roman times, as a soap making factory was found in the ruins at Pom-
peii. Also, the word
sapo
, Latin for soap, was first reported by Pliny the Elder in his great
work
[2]
. Pliny described the making of soap from tallow and ashes. The
origin of the word saponification is most interesting. It has been suggested that it comes
from Sapo Mountain near Rome. According to Roman legend from about 1,000 BC, women
washing clothes in the Tiber River noticed that their clothes were cleaner if they washed them
in a particular area. On the mountain above the special spot in the river was found a place
where animals were sacrificed on an altar. The animal fat was inadvertently mixed with
heated ashes and lye, producing a crude soap that washed into the Tiber. Hence the term
saponification! Although this makes for a great story that for many years has been propa-
gated by soap manufacturers, it is probably only a myth, as Sapo Mountain is in fact a ficti-
tious place. Also, the first archeological evidence of soap manufacturing came from ancient
Babylon (2,800 BC), and a formula for soap consisting of water, alkali, and cassia oil was
written on a Babylonian clay tablet around 2,200 BC
[3]
.
While many fatty acyl chains are attached to lipids and proteins by hydrolyzable ester or
amide bonds, others are attached by non-hydrolyzable ether linkages. Ether bonds are partic-
ularly prevalent in very primitive organisms including Archaea. Archaea live in extreme
conditions where membrane ester linkages would be highly susceptible to hydrolysis.
They characteristically have long chain (32 carbons), branched hydrocarbons linked at
each end via ether linkages to glycerols that have either phosphate or sugar residues
attached. These unusual lipids are twice the width of regular membrane phospholipids
and so can span the bilayer while being anchored in both internal and external aqueous
spaces. Another curiosity of these membrane lipids is that the glycerol central carbon isomer
is the opposite of that found in phospholipids (discussed below). It is interesting to note that
while primitive organisms have stable ether linkages, higher organisms have emphasized
less stable ester linkages. Esters can be cleaved by saponification (chemical hydrolysis) or
by the action of phospholipases (enzymatic cleavage, discussed below), while ethers are
resistant to both processes.
In man one unusual phospholipid class called plasmalogens
[4]
have both an ester-linked
acyl chain as well as an ether linked chain (for the structure of plasmalogens see
Figure 5.4
).
This raises the intriguing possibility that the ether linkage found in plasmalogens perhaps are
a vestige held over from an ancient past. For many years plasmalogens and other ether lipids
were considered little more than biological curiosities. However, their large abundance in
nature indicates ether-lipid bonds must play significant roles in life processes, particularly
Historia Naturalis