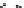Biology Reference
In-Depth Information
H
H
H
H
H
H
H
C
CCCCC
H
H
H
H
H
H
H
HH
Interface Water
2 H-Bonds
O
H
H
H
H
O
O
O
HH
O
H
H
Bulk Water
4 H-Bonds
O
HH
O
HH
O
HH
FIGURE 3.5
Dispersion of hexane into water is highly unfavorable due to the difference in H-bonds available to
bulk-waters (4) compared to hexane-interface waters (2).
to the interfacemakes thebulkwater structuremore thermodynamically favorable.As a general
rule, the more H-bonds that can be formed, the more stable the structure will be. Therefore
water molecules at the air
water interface feel a net force of attraction that pulls them away
from the interface and back into the bulk of the liquid. As a result the entire liquid tries to
take on a shape that has the smallest possible surface contact area with air or oil
e
a sphere.
Thus, ignoring gravitational effects, the shape of a raindrop would be a sphere. The stronger
theH-bonds betweenmolecules, thehigherwill be the surface tension. The intermolecular force
of adhesion between water and a hydrocarbon that comprise the interior of a membrane is
much lower than the intra-molecular force of cohesion between the water molecules them-
selves. Therefore water tries to minimize its surface contact area by separating from the hydro-
carbon. This is known as the Hydrophobic Effect
[10,11]
and is the major stabilizing force for
membrane structure (discussed below). Hydrophobic means fear of (phobia) water (hydro).
Surface tensionwas one of the first quantitativemeasurements that was possible on a 'model
membrane' and so was heavily employed by the early monolayer experimental physicists
including Agnes Pockels and Irwin Langmuir (Chapter 2). Earliest measurements were done
employing a Du Nouy Ring, named after the French physicist who developed the technique
in the late 1890s. Actually, Agnes Pockels predated Du Nouy by using common shirt buttons!
By this method, a platinum ring (now replaced by a platinum-iridium alloy) is attached to
a sensitive tensiometer incorporating a precision micro-balance. The ring is immersed in the
aqueous solution just beneath the air
e
water interface and the force required to pull the ring
slowly through the interface is measured (
Figure 3.6
). The force slowly increases until
amaximumvalue is reached.At thispoint, the ringbreaks free fromthe surface, leaving the solu-
tion, and the pulling force drops to zero. Surface tension is directly related to the maximal force
required to free the ring from the solution. A similar method in use today employs a Wilhelmy
e