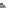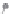Biology Reference
In-Depth Information
detergent. Besides being separated into these five classes, each detergent is given two
numbers that may be predictive of its use in membrane studies
the critical micelle concen-
tration (CMC) and the hydrophile/lipophile balance (HLB). CMC is an actual, measured
parameter while the HLB is an empirically derived number.
The CMC is the detergent (or any surfactant) concentration above which micelles are spon-
taneously formed
[2]
. Below the CMC each detergent molecule will either reside at the
aqueous interface or, if water soluble enough, be solvated. Above the CMC additional surfac-
tant will only increase the number of micelles in solution. At the CMC, micelles form,
changing several basic parameters of the solution. The abrupt solution changes that occur
at the CMC are commonly followed by an increase in solution turbidity (light absorbance),
a change in electrical conductivity or a change in surface tension. Determination of CMC
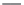
using surface tension measurements is shown in
Figure 13.2 [3]
. At low concentrations, the
detergent will preferentially accumulate at the air/water interface greatly reducing the
surface tension. As detergent accumulates at the interface, the increasingly crowded condi-
tion forces some detergent into the aqueous sub-phase, resulting in the generation of
micelles. The detergent concentration where this occurs is the CMC. CMCs for some common
membrane detergents are listed in
Table 13.1
.
A second number often used to characterize a detergent is the HLB (hydrophile/lipophile
balance)
[4,5]
. The HLB is a number that is an empirical measure of how hydrophilic or
hydrophobic the detergent is. There are several ways to calculate the HLB. The most
commonly used method to calculate HLBs for membrane studies was developed by Griffin
in papers published in 1949 and 1954
[4,5]
. The Griffin method calculates the net HLB from
e
Surface tension
CMC
Concentration
FIGURE 13.2
Determination of a detergent's CMC by surface tension measurements. At low concentrations, the
detergent will preferentially accumulate at the air/water interface greatly reducing the surface tension. As deter-
gent accumulates at the interface, the ever increasing crowded condition forces some detergent into the bathing
solution generating micelles at the CMC. At this point there is no further decrease in surface tension with additional
detergent
[3]
.