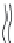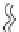Biology Reference
In-Depth Information
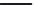
distearoyl PC (DSPC 18:0,18:0 PC). Bacteriorhodopsin-induced shifts in the expected T
m
of
each lipid were determined by DSC. Shifts in T
m
s were consistent with Mouritsen and
Bloom's 'Mattress Model' (see
Figure 10.23
, right panel)
[69]
. According to this model,
a protein with a larger hydrophobic match length than the solvating lipid can stretch the lipid
to fit its hydrophobic segment. This results in an increase in lipid order that is associated with
an increase in the lipid's T
m
. In contrast, a protein with a shorter hydrophobic match length
than the solvating lipid can shrink the lipid to fit its hydrophobic segment. This results in
a decrease in lipid order that is associated with a decrease in the lipid's T
m
. Tocanne found
that bacteriorhodopsin reconstituted into DLPC bilayers increased the lipid's T
m
þ
40
C
(from 0
C to ~40
C) (
Figure 10.23
, left panel). Therefore, the length of DLPC (2.4 nm) is
shorter than the hydrophobic match length of bacteriorhodopsin. The T
m
of DMPC was
also increased by bacteriorhodopsin, but only by
23
C (from 23.6
C to ~46.6
C). Therefore,
the length of DMPC (2.8 nm) is also shorter than the hydrophobic match length of bacterio-
rhodopsin. Upon incorporation of bacteriorhodopsin into the longer lipid DSPC (3.7 nm), T
m
of the lipid decreased from 58
Cto45
C(
þ
13
C). This indicates that the hydrophobic match
length must be shorter than 3.7 nm (DSPC), but longer than 2.8 nm (DMPC). When bacterio-
rhodopsin was incorporated into DPPC bilayers (3.2 nm), no shift in T
m
(41.3
C) was
observed. This indicates that the length of the hydrophobic match for bacteriorhodopsin
is ~3.2 nm. Other methodologies agree, placing the hydrophobic match length for
50
d
L
d
p
30
A
10
-10
d
p
d
L
-30
20
25
30
35
40
Mean hydrophobic thickness
d,
Å
B
FIGURE 10.23
The Mouritsen and Bloom 'Mattress' model
[69]
(right panel). The hydrophobic match length of
an integral protein is depicted by d
P
, and the length of the lipid bilayer hydrophobic interior by d
L
.Ifd
P
>
d
L
, the
lipid must stretch to match the protein hydrophobic length, thus increasing its T
m
(right panel, part A). If d
P
<
d
L
,
the lipid must shrink to match the protein hydrophobic length, thus decreasing its T
m
(right panel, part B). This
principle was tested by Toconne and co-workers
[68]
for bacteriorhodopsin reconstituted into bilayers made from
either DLPC (12:0,12:0 PC), DMPC (14:0,14:0 PC), DPPC (16:0,16:0 PC) or DSPC (18:0,18:0 PC) (left panel). The
bacteriorhodopsin-induced change in expected T
m
s for each lipid was determined by DSC and plotted against
the mean hydrophobic thickness. Bacteriorhodopsin reconstituted into DLPC and DMPC bilayers increased the
expected T
m
and so both lipids are shorter than the protein's hydrophobic match. Bacteriorhodopsin reconstituted
into DSPC bilayers decreased the expected T
m
and so is longer than the protein's hydrophobic match. Bacterio-
rhodopsin had no effect on the T
m
of bilayers made from DPPC. Therefore bacteriorhodopsin's hydrophobic match
length is about the same as the hydrophobic length of DPPC, ~30.5
˚
[68]
.