Biology Reference
In-Depth Information
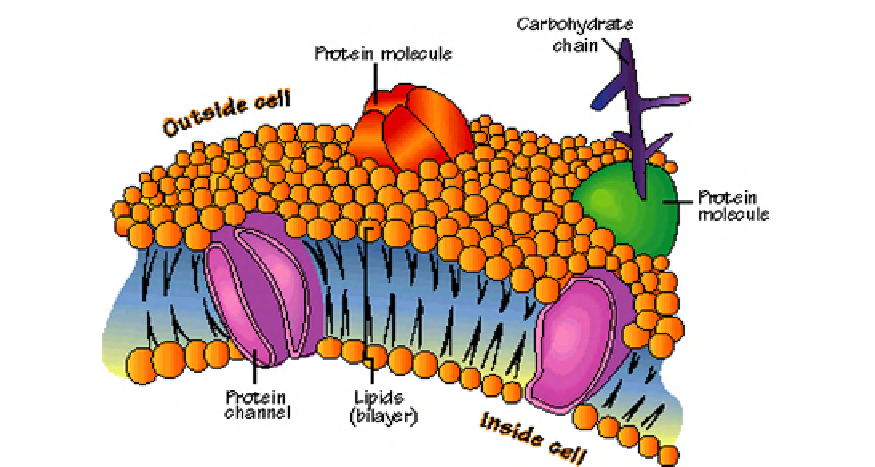
FIGURE 10.19
An illustration of the components of a 'typical' plasma membrane. Similar drawings are
commonly found in many textbooks. Note the unrealistically high number of phospholipid shells surrounding each
protein (~11 lipid rings). Courtesy of ThinkQuest.
crowded. Despite this, the activity of many membrane proteins is known to be significantly
affected by specific lipids
[45
47]
. Annular lipid selectivity is weak and may reside with
either the lipid head group or hydrophobic tails. However, it does appear that at least
some integral proteins, in addition to the weakly associated annular lipids, also have
a few binding sites for specific lipids. These are called 'non-annular' or 'co-factor' lipids.
The best example of strong preferential affinity for membrane proteins has been demon-
strated for anionic lipids binding to the Ca
2
þ
ATPase, Protein Kinase C, Na
þ
/K
þ
ATPase,
and the Potassium Channel KcsA.
e
Ca
2þ
ATPase
One integral membrane protein that has contributed substantially to understanding lip-
protein interactions is the Ca
2
þ
ATPase, a protein that is easily isolated from the sarco-
plasmic reticulum of skeletal muscle. Discovery of the enzyme and its function, to pump
Ca
2
þ
out of the sarcoplasmic reticulum, dates from the early 1960s. One advantage of this
enzyme is that it has two measurable activities, trans-membrane Ca
2
þ
transport and ATP
hydrolysis. Importantly, the enzyme can be reconstituted using a deoxycholate dialysis
procedure (discussed in Chapter 13).
In a 1975 paper in Nature, Warren et al. reconstituted the Ca
2
þ
ATPase into liposomes made
from a variety of lipid mixtures
[43]
. When these investigators plotted PC content versus
enzyme activity they estimated that activity returned at a PC-protein ratio of ~30
(
Figure 10.18
). They interpreted this number as an estimate of the number of lipids required
to completely surround the Ca
2
þ
ATPase once (hence the size of the annular lipid boundary).
id
e