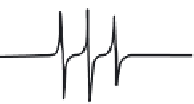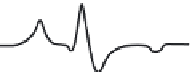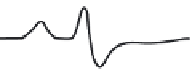Biology Reference
In-Depth Information
Initially, electron spin resonance (ESR) of spin-labeled phospholipids was used to distin-
guish annular from bulk bilayer lipids
[39]
. The approach is based on motional restriction of
any spin probe-lipid that is adjacent to an integral protein (annular lipids). Restricted
motion results in a much broader ESR spectrum than is measured for freely rotating
spin-labeled phospholipids (bulk bilayer lipids,
Figure 10.17
)
[40]
. Therefore, addition of
a spin-labeled phospholipid to a membrane containing protein(s) could produce a two
component spectrum if there is a motional difference between annular and bulk bilayer
lipids that exists for at least 10
8
10
7
sec, the shortest time scale detectable by ESR. A
two component spectrum was indeed detected by ESR, suggesting the possible existence
of annular lipids. This was corroborated by use of another rapid time technique, fluores-
cence quenching. Brominated phospholipids
[41]
were used to quench the inherent fluores-
cence of tryptophans in the integral protein. Fluorescence quenching is very sensitive to
distances on the nm range. Brominated annular lipids, being closer to the tryptophans,
e
Description of
spectra
Approx, rotational
lumbling times (ns)
0-1
Freely lumbling
43ºC
0-6
26ºC
Weakly
immobilized
2-5
9ºC
Moderately
immobilized
5-0
0ºC
Strongly
immobilized
-300
- 36ºC
Fluid glass
or powder
>300
- 100ºC
0.5mT
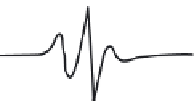
FIGURE 10.17
ESR spectra of a nitroxide spin probe from freely tumbling (top) to totally restricted (glass
state, bottom). Note the freely tumbling spectrum is narrow and sharp while motional restrictions broaden the
spectra.