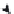Biology Reference
In-Depth Information
2
4
(a)
1
3
(b)
rotate 60˚
rotate 60˚
H
H
H
H
4
H
H
H
3
3
3
2
2
2
4
H
H
1
H
1
1
H
H
4
TRANS
ECLIPSED
GAUCHE
(c)
Gauche
Kink
GAUCHE
TRANS
FIGURE 9.21
Melting of a saturated fatty acyl chain. Part (a) depicts a 4-carbon section somewhere along
a saturated acyl chain existing in the un-melted trans state. Part (b) demonstrates Newman projections for the 4
sequential carbons depicted in (a). Upon heating the chain, rotation occurs around carbons 2 and 3. The trans
configuration has the lowest possible energy (part c) as the largest groups, carbon 1 and carbon 4, are as far apart as
possible. As the large C-4 passes a H, steric hindrance is encountered as depicted in the eclipsed state. The eclipsed
state is an unfavorable, high-energy transient state (part c). Further rotation relieves some of the steric stress,
resulting in another low energy state referred to as the gauche state. The gauche state is of lower energy than the
eclipsed state, but higher energy than the trans state, since there is more steric interference between the closer, bulky
C-1 and C-4 groups. This lower energy state creates a 'gauche kink' in the chain (part c). It is the accumulation of
'gauche kinks' in the chain that results in chain melting.
there is more steric interference between the closer, bulky C-1 and C-4 groups. This lower
energy state creates a 'gauche kink'inthechain(
Figure 9.21
c, far right). It is the accumu-
lation of 'gauche kinks' in the chain that results in melting. At 37
C there are ~2 kinks per
acyl chain and the chain exists in the melted, liquid crystalline state. An acyl chain is essen-
tially tethered to a bulky head group. The kinks then rapidly (~10
9
sec) work their way
down the chain and are released at the bilayer interior. For this reason acyl chains exhibit
minimal motion near the polar head and maximal motion at the methyl chain terminus
(the omega end). Therefore, an acyl chain is more fluid at its omega end than at its alpha
end.