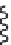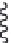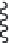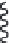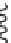Biology Reference
In-Depth Information
protein
LIPID FLIP-FLOP
(unassisted)
LIPID FLIP-FLOP
(protein-assisted)
FIGURE 9.18
Lipid flip-flop, unassisted (left) and protein-assisted (right). A protein's surface can increase
phospholipid flip-flop rate 10
3
to 10
5
fold.
asymmetry could not be maintained. If flip-flop was very slow, a lipid's asymmetry would
reflect the membrane sidedness during its synthesis and it would be hard to explain how
each membrane has a different asymmetry for each lipid. The well-established fact that all
membranes exhibit partial lipid asymmetry indicates that the process of flip-flop might
not be easy, but it should be possible. As a lipid undergoes flip-flop, two thermodynamically
unfavorable events must occur simultaneously. The lipid hydrophobic tails must be exposed
to water and the polar head group must be exposed to the hydrophobic bilayer interior. From
this a rough estimate of the energy of flip-flop can be made. It requires ~2.6 kcal of free energy
to transfer a mole of methane from a nonpolar medium to water at 25
C. Free energy required
to transfer a mole of zwitterionic glycine from water to acetone is about 6.0 kcal at 25
C.
Therefore it would very roughly require approximately 97 kcal to flip a phospholipid across
a membrane (assuming an average chain length of 17.5 carbons per chain, and two acyl
chains per phospholipid). This means that only 1 phospholipid in ~10
15
would have enough
energy to flip and so the inherent rate of flip-flop would be quite slow, but not impossible.
Indeed this has been shown to be the case
[27]
. This slow rate can, however, be substantially
increased (10
3
to 10
5
fold) by simply providing a trans-membrane protein surface
(
Figure 9.18
) [28 for glycophorin and
[29]
for cytochrome b5]. Although lateral exchange of
nearest lipid neighbors in the same membrane leaflet, and exchange across the membrane
from one leaflet to another, traverse approximately the same distance (~4 nm), the lateral
exchange rate is ~10
10
x faster than is flip-flop. Nevertheless, lipid flip-flop is an essential
component of membrane dynamics. Importance of flip-flop begins with biogenesis of the
phospholipid and ends with cell death
[30]
.
Chemical Method to Determine Flip-Flop Rates
Since flip-flop is essentially the kinetics of asymmetry, any method that can be used to
measure lipid asymmetry should, in principle, also be applicable to measuring flip-flop.
For example, the rate of flip-flop of the primary amine phospholipids PE and PS can be deter-
mined by combining the methods discussed above using trinitrobenzene sulfonate (TNBS)
and isethionylacetimidate (IAI) for determining lipid asymmetry. The structure of these
compounds is shown in
Figure 9.8
and their application for determining flip-flop in
Figure 9.19
. If a sealed membrane vesicle (LUV) is exposed to TNBS, only the outer leaflet