Biology Reference
In-Depth Information
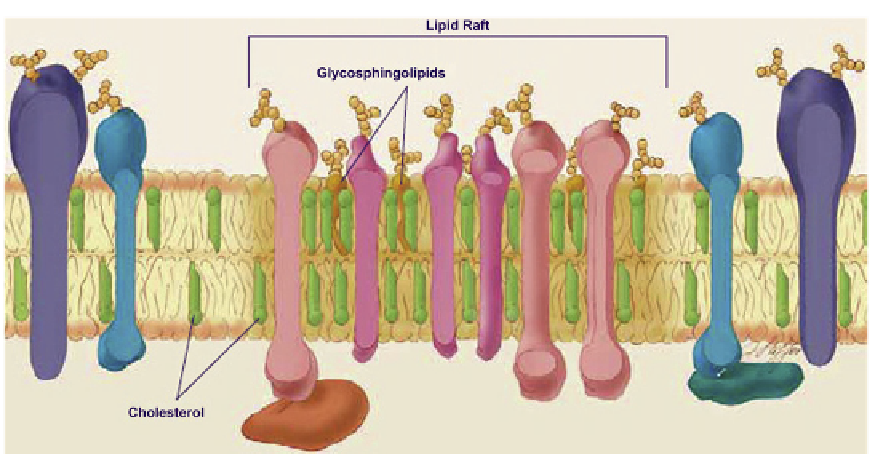
FIGURE 8.8
Simons' Lipid Raft model. Reprinted with permission
[30]
.
(ordered, solid) state. These states are discussed in detail in Chapter 9. Briefly, the acyl chains
of lipid rafts are more saturated than are the acyl chains of the surrounding membrane matrix
and so pack more tightly, producing the l
o
state. It is believed that lipid rafts are held together
by cholesterol, reflecting the sterol's high affinity for sphingolipids. Typically, raft cholesterol
levels are twice that found in membrane non-raft regions, while the SM concentration is
~50% higher and the PC level is reduced in proportion to the increase in SM. As a result
of the tight packing, lipid raft portions of the membrane are thicker than the surrounding,
more loosely packed membrane, and so protrude above the surrounding membrane surface.
Lipid rafts are believed to house a variety of raft-characteristic proteins that are intimately
involved in cell signaling events. Often these proteins affect the phosphorylation state of
signaling proteins that can be modified by local kinases and phosphatases to affect down-
stream signaling.
An original hallmark of lipid rafts is their ability to be isolated from the remaining
membrane matrix by extraction in cold (4
C) non-ionic detergents (e.g. Triton X-100 or
Brij-98). Under cold, detergent conditions, the membrane matrix is solubilized while the lipid
rafts are insoluble (they are detergent resistant). Because of their composition and detergent
resistance, lipid rafts are also referred to as detergent-insoluble glycolipid-enriched
complexes (GEMs or DIGs) or detergent resistant membranes (DRMs). It is believed,
although by no means proven, that lipid rafts are normally extremely small (~10
200nm)
but their size can be greatly enhanced by various natural and artificial cross-linking agents.
In a 2006 Keystone Symposium on Lipid Rafts and Cell Function
[26]
, lipid rafts were
defined as 'small (10
e
200nm), heterogeneous, highly dynamic, sterol- and sphingolipid-
enriched domains that compartmentalize cellular processes. Small rafts can sometimes be
stabilized to form larger platforms through protein
e
protein interactions'.
e