Agriculture Reference
In-Depth Information
30
7.00
30
25
25
HCO
3
−
CO
2
pressure
6.75
20
20
15
6.50
15
B
2
+
pH
10
10
Fe
2
+
6.25
5
A
+
5
0
6.00
0
0
20
40
60
80
100
Fe(III) reduced (mmol kg
−
1
)
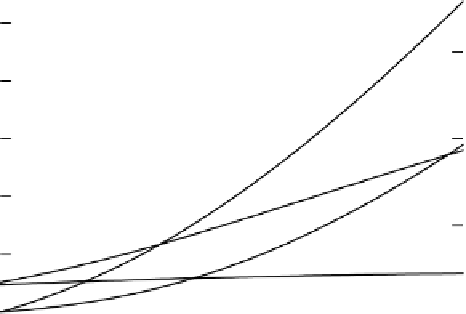
Figure 3.17
Calculated changes
in a
soil
solution upon reduction of Fe(III)
oxide
coatings on soil
surfaces
and structural Fe(III)
in clay lattices with-
re-precipitation of Fe(II). A
+
and B
2
+
out
are exchangeable cations. Param-
[A
+
]
=
0
.
1[B
2
+
], CEC
0
=
100mmol kg
−
1
,
eter values
in Equations (3.60)-(3.72):
5mmol
c
L
−
1
,pH
0
=
50mmol pH
−
1
kg
−
1
,
K
E1
[X]
L
=
6,
b
HS
=
=
1,
K
E2
=
1,
ψ
=
0
.
5,
m
=
0
.
5,
n
=
0
.
75
,θ/ρ
=
0
.
7
Figure 3.17 shows changes in the composition of a simulated soil solution so
calculated. The proportions of Fe reduced in oxide coatings and structural Fe
are equal. The figure shows that as Fe
2
+
accumulates and the CEC, pH and
[HCO
3
−
]
L
increase, the concentrations of Fe
2
+
and B
2
+
in solution increase, but
the concentration of the monovalent A
+
in solution changes less because it is
more poorly buffered by the exchange complex. With a greater proportion of
structural Fe reduced, less Fe
2
+
accumulates and the increase in pH is smaller
but nonetheless the CEC increases and so the increases in B
2
+
in solution with
oxide dissolution and accumulation of HCO
3
−
is smaller.