Agriculture Reference
In-Depth Information
Oxygen
Metal
H
H
Precipitated
metal
H
H
Solid
−
water
interface
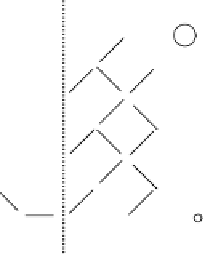
Figure 3.14
Precipitation on the surface of an oxide or edge of a layer silicate (cf.
Fig. 3.11)
Table 3.14
Rates of solid-solution interactions in soils
Process
Time scale
(h)
10
−
8
-10
Complexation in solution
Adsorption
10
−
8
-10
10
−
6
-10
4
Desorption
1-10
4
Dissolution
10
−
6
-10
8
Redox reactions in solution
0
.
1-10
4
Redox reactions on surfaces
Solid solution formation
0.1-10
10
−
2
-1
Cluster formation
1-10
2
Heterogeneous nucleation and precipitation
Homogeneous nucleation and precipitation
0
.
1-10
4
10
4
-10
8
Recrystallization into pure phases
10-10
8
Diffusion on surfaces
10
5
-10
8
Diffusion in crystals
Thus rate laws for precipitation reactions tend to be complicated, even in pure
solutions. Mixed precipitates can be inhomogeneous solids with one component
restricted to a thin outer layer because of slow diffusion. New solid phases can
precipitate homogeneously onto the surfaces of existing solid phases. Weathering
solids may provide host surfaces onto which more stable phases may precipitate.
At least three potentially rate-limiting steps can be distinguished: the diffusion
of the reacting solutes to the site of precipitation; their reaction to form the
insoluble compound; and accumulation of the compound as a solid phase. In