Agriculture Reference
In-Depth Information
(a) [Alk]
10 mM
=
40
1.0
9.0
HCO
3
−
/C
T
8.5
0.8
Free CO
2
30
pH
8.0
0.6
20
7.5
0.4
7.0
10
0.2
CO
3
2
−
/C
T
H
2
CO
3
*
/C
T
6.5
0.0
0
6.0
(b) [Alk]
=
0.5 mM
40
1.0
9.0
Free CO
2
HCO
3
−
/C
T
8.5
0.8
30
8.0
0.6
pH
20
7.5
0.4
7.0
H
2
CO
3
*
/C
T
10
0.2
6.5
0.0
0
6.0
6
8
10
12
14
16
18
20
22
Time (h past midnight)
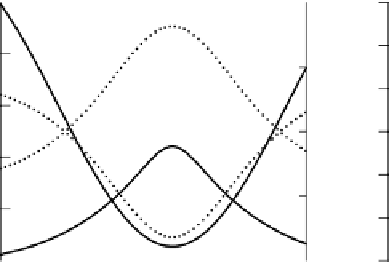
Figure 3.3
Calculated diurnal changes in the pH and concentrations of carbonate
species in ricefield floodwater for sinusoidally varying [H
2
CO
3
∗
] with (a) [Alk]
=
10mM,
(b) [Alk]
=
0
.
5mM. The free CO
2
concentrations are in mg L
−
1
to be consistent with
Figure 3.2
caused by additions of nitrogenous fertilizers. Effects on pH again depend on
initial pH and corresponding buffer systems operating.
3.4 GAS TRANSPORT ACROSS THE AIR-WATER INTERFACE
The floodwater is for the most part not in equilibrium with the atmosphere
because rates of production of volatile solutes in the water exceed rates of gas
exchange across the air-water interface. In particular, during the day, rates of
CO
2
consumption and O
2
production by photosynthesizing organisms are gen-
erally sufficient to cause undersaturation of CO
2
and supersaturation of O
2
.
Conversely, at night, respiration causes depletion of O
2
and supersaturation of
CO
2
. The underlying soil is also a large sink for O
2
and source of CO
2
.The
resulting diurnal changes in dissolved CO
2
can cause large changes in floodwater
pH, often from near neutral at night to pH 10 during the day.