Agriculture Reference
In-Depth Information
100
10
60
HCO
3
−
90
50
9
pH
40
80
8
15
30
10
20
Free CO
2
7
CO
3
2
−
H
2
CO
3
5
10
0
0
6
600
800 1000 1200 1400 1600 1800 2000 2200
Time
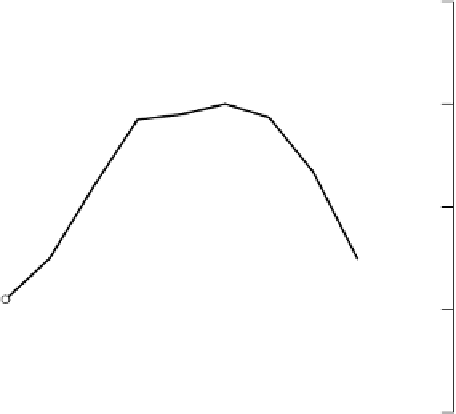
Figure 3.2
Diurnal changes in pH and concentrations of carbonate species in the flood-
water in a ricefield (Mikkelsen
et al
., 1978). Reproduced by permission of Soil Sci.
Soc. Am.
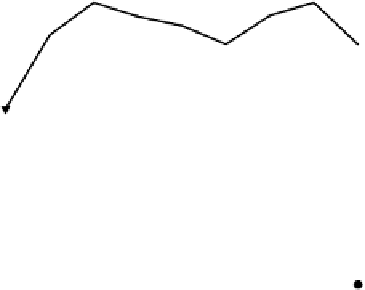
and the pH change is correspondingly larger again. Figure 3.3 shows calculated
changes in pH for a sinusoidally varying floodwater [H
2
CO
3
∗
] over the day
for two different alkalinities. The dissolved CO
2
concentrations are the same in
Figure 3.3(a) and (b); only the alkalinities differ.
In principle, the alkalinity of the water will also be affected by the balance
of nutrient ions consumed and released by organisms in the water. But in prac-
tice these have a minor affect compared with CO
2
. The average composition of
the algal biomass in natural waters is given by the Redfield formula (Redfield,
1934) as C
106
H
263
O
110
N
16
P. Therefore for the complete stoichiometry of algal
photosynthesis and respiration, we have with NO
3
−
as the source of N
106CO
2
+
16NO
3
−
+
H
2
PO
4
−
+
122H
2
O
+
17H
+
=
C
106
H
263
O
110
N
16
P
+
133O
2
(3.25)
and with NH
4
+
106CO
2
+
16NH
4
+
+
H
2
PO
4
−
+
106H
2
O
=
C
106
H
263
O
110
N
16
P
+
106O
2
+
15H
+
(3.26)
The corresponding changes in alkalinity are
+
17
/
106
=+
0
.
16mol
c
per mol C
fixed for NO
3
−
nutrition and
−
15
/
106
=−
0
.
14mol
c
per mol C fixed for NH
4
+
nutrition. More significant changes in the alkalinity of ricefield floodwater are