Agriculture Reference
In-Depth Information
Since [HB] and [HS] are both functions of pH, and pH is a function of
x
,
∂
[HB]
∂x
∂
[HS]
∂x
d[HB]
dpH
dpH
d[HS]
=
b
HB
b
HS
∂
[HS]
∂x
=
Hence substituting in Equation (2.27),
θ
L
f
L
b
HS
b
HB
D
LHB
∂
[HS]
∂x
∂
[HS]
∂t
∂
∂x
=
(
2
.
31
)
where the term in parentheses is the soil acidity diffusion coefficient,
D
HS
.If
b
HS
is constant, then by substituting for d[HS] from Equation (2.30), Equation (2.31)
may be written
D
HS
∂
pH
∂x
∂
pH
∂t
∂
∂x
=
(
2
.
32
)
For a soil in which the only important acid-base pairs are H
3
O
+
-H
2
Oand
H
2
CO
3
-HCO
3
−
, Nye (1972) shows that:
2
.
303
θ
L
f
L
b
HS
(D
LH
[H
3
O
+
]
+
D
LC
[HCO
3
−
]
)
D
HS
=
(
2
.
33
)
The relative contribution of the pairs H
3
O
+
-H
2
OandH
2
CO
3
-HCO
3
−
to the
overall soil acidity diffusion coefficient is given by the term in parentheses in
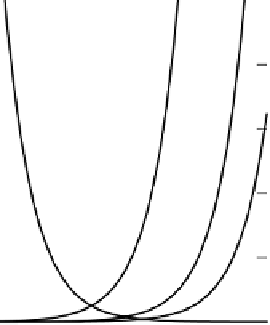
Equation (2.33) and is plotted at different pHs in Figure 2.9(a). The figures shows
H
3
O
+
--- H
2
O
H
2
CO
3
---HCO
3
−
(a)
(b)
1.0
1.0
P
CO
2
0.05 kPa
=
0.8
P
CO
2
1 kPa
=
0.8
0.6
0.1
0.4
0.6
0.03
0.2
0.0
0.4
345678
pH
0.2
0.0
4
5
6
7
8
pH
Figure 2.9
(a) Contributions of acid-base pairs H
3
O
+
-H
2
OandH
2
CO
3
-HCO
3
−
to the
soil acidity diffusion coefficient over a range of pH;
θ
L
f
L
=
0
.
3
,b
HS
=
0
.
05mol dm
−
3
pH
−
1
(after Nye, 1972). (b) Observed and calculated soil acidity diffusion coefficients (Nye and
Ameloko, 1986). Reproduced by permission of Blackwell Publishing