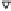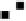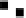Agriculture Reference
In-Depth Information
(a)
100
(b)
100
90
4.5
4.5
30
5.0
75
10
5.0
5.5
6.0
60
3
1
6.0
45
6.5
0.3
6.5
0.1
30
0
50
100
150
200
250
0
50
100
150
200
250
Time (min)
Time (min)
(c)
90
75
6.0
60
45
30
5.0
4.5
15
0
50
100
150
200
250
Time (min)
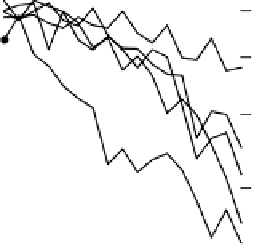
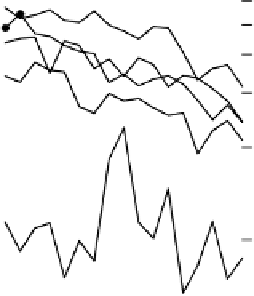
Figure 4.16
Changes in concentrations of Fe
2
+
in (a) whole soil, (b) soil solution and
(c) soil solid during oxygenation of reduced soil suspensions at different pHs. [Fe
2
+
]
S
was
calculated from [Fe
2
+
]-
R
[Fe
2
+
]
L
(Kirk and Solivas, 1994). Reproduced by permission
of Blackwell Publishing
A possible explanation is that access of O
2
to the exchange or Fe(OH)
3
sites
where the Fe
2
+
is adsorbed is restricted. Possibly Fe(OH)
3
is precipitated between
clay lamellae at the oxidation sites and it partially blocks the original exchange
sites. This mechanism would also imply a wide range of reaction rates between
soils, with
k
S
values ranging by perhaps an order of magnitude, as in Figure 4.15.
In summary, the reaction can be represented by the following simplified scheme:
k
S
−−−→
Fe(OH)
3
Fe
2
+
L
Fe
2
+
S