Agriculture Reference
In-Depth Information
4.4 OXIDATION OF REDUCED SOIL
When a spadeful of wet, anaerobic soil is brought to the surface and allowed
to dry, air enters through drying cracks and the soil tends to become uniformly
oxidized and turn a uniform brown. Whereas when oxidation occurs without
drying — as, for example, near a root releasing O
2
into wet soil — it is far less
uniform and reddish-brown ferric oxide deposits form on and near the oxidizing
source. The difference depends on the relative rates of movement of O
2
into the
soil and of ferrous iron and other reductants in the opposite direction, and the
rates of reaction.
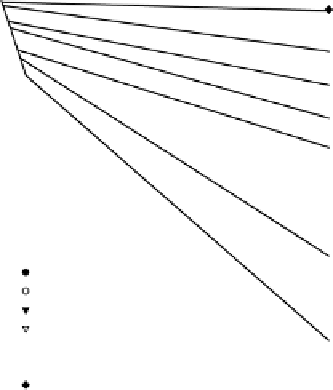
Figure 4.15 indicates the range of rates of O
2
consumption in different soils.
Oxygen is consumed in oxidation of inorganic reductants, such as Fe(II), as
well as in oxidation of organic matter by microbes. Bouldin (1968) and Howeler
and Bouldin (1971) compared measured rates of O
2
movement into anaerobic
soil cores with the predictions of various models, and obtained the best fits
with a model allowing for both microbial respiration and abiotic oxidation of
mobile and immobile reductants; abiotic oxidation accounted for about half the
O
2
consumed. The kinetics of the abiotic reactions are complicated. They often
depend on the adsorption of the reductant on solid surfaces as, for example, in
1
0.1
pH Org C [Fe
2
+
]
(%) (
mol g
−
1
)
µ
6.2 1.60 42.3
6.6 2.30 39.6
5.9 0.82 33.6
6.8 1.71 18.9
5.6 0.72 26.9
5.6 1.01 5.3
7.6 0.54 16.5
0.01
0
20
40
60
80
Time (h)
Figure 4.15
Rates of oxygen consumption by shaken suspensions of anaerobic soils.
Points are measured data, lines are fits to two first-order rate equations. The apparent
rate constant for the initial reaction is common to all soils; that for the main reaction
varies 30-fold between the soils and is well correlated with [Fe
2
+
] (Reddy
et al
., 1980).
Reproduced by permission of Soil Sci. Soc. Am.