Agriculture Reference
In-Depth Information
The initial increase occurs because SO
4
2
−
sorbed on variable charge clays and
oxides is desorbed as the pH increases. The rate of subsequent reduction will be
low if the pH remains below 5.5, the optimal range of pH for SO
4
2
−
reducing
bacteria being greater than this.
4.3.4
TRANSFORMATIONS OF PHOSPHORUS
Phosphorus is often the most limiting nutrient in natural wetlands. Because of
its association with soil Fe, its solubility changes markedly during reduction and
oxidation. In general it is not itself reduced and remains in the
+
5 oxidation
state, though production of phosphine gas (PH
3
;
+
3 oxidation state) at rates
≤
6
.
5ngm
−
2
h
−
1
has been reported in laboratory experiments with brackish and
saline marsh soils (Devai and Delaune, 1995). Review articles on transformations
of P in submerged soil include Patrick and Mahapatra (1968), Kirk
et al
. (1990a)
and Willett (1991).
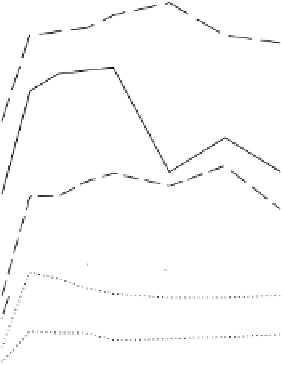
Typically when a soil is submerged the concentrations of water- and acid-
soluble P increase, reach a peak or plateau, and then decrease (Figures 4.11c and
4.13). For the soils shown in the figures, the peak P concentrations in solution
were smallest for acid soils high in active Fe and greatest for a sandy soil low in
Fe. The increases in acid-soluble P were greatest in an alkali soil low in active
1.2
1.0
26
0.8
0.6
27
18
0.4
21
0.2
28
14
0.0
0
2
4
6
8
10
12
Time (weeks after submergence)
Figure 4.13
Changes following flooding in the concentration of P soluble in an acetate
buffer at pH 2.7. Numbers next to curves identify soils; properties given in table in
Figure 4.11 (modified from Ponnamperuma, 1985). Reproduced by permission of IRRI