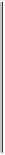Agriculture Reference
In-Depth Information
NO
3
−
+
5
12
4
+
NO
2
+
3
NO
2
−
+
2
NO
pe
+
1
N
2
O
0
N
2
N fixation
−
4
i
mmobiliza
tion
−
3
NH
4
+
Organic N
mineralization
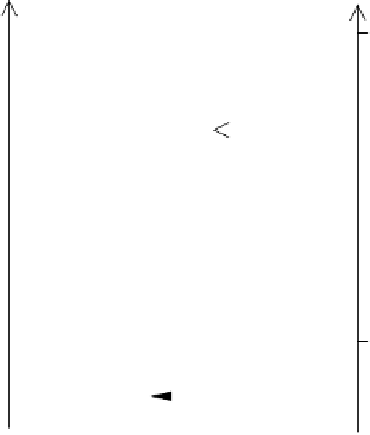
Figure 4.10
Nitrogen transformations in submerged soils on a redox scale (McBride,
1994). Reproduced by permission of Oxford University Press
accumulates in the soil solution and exchange complex. Because of the low N
requirement of anaerobic metabolism, subsequent immobilization by microbes
tends not to be important, or, if it occurs — as when organic matter with a wide
C:N ratio is present — the immobilization is temporary.
Further transformations of N take place at the oxic interfaces between the soil
and floodwater and root and soil where NH
4
+
diffusing in from the neighbouring
anoxic soil may be nitrified to NO
3
−
. Subsequently, NO
3
−
diffusing out into the
anoxic soil may be denitrified to N
2
. This process results in significant losses of
N from wet soils but its importance in submerged soils is unclear (Section 5.3).
Under strongly reducing conditions
(
pe
<
−
4
)
reduction of N
2
to NH
4
+
is
thermodynamically possible. The net reaction is
3
H
+
+
3
NH
4
+
+
1
1
1
1
1
6
N
2
(
g
)
+
4
'CH
2
O'
=
4
CO
2
(
g
)
=−
14
.
3kJmol
−
1
at pH 7. However this reaction has a very large acti-
vation energy because of the energy required to break the N
≡
N triple bond
(
942 kJ mol
−
1
)
. Therefore only highly specialized 'nitrogen fixing' organisms are
capable of maintaining sufficiently reducing conditions in their cells to mediate
the reaction. The niches in submerged soils in which nitrogen fixers may operate
are discussed in Chapter 5.
Most of the mineralizable N in the soil is converted to NH
4
+
within a few
weeks of submergence if the temperature is favourable and the soil not strongly
acid or deficient in other nutrients. The concentration of NH
4
+
in the soil solution
typically reaches 0.1 to 5 mM buffered by from 5 to 20 times this concentration
G
o