Agriculture Reference
In-Depth Information
(a)
20
(b)
(c)
(d)
(e)
P
O
2
> 1 atm
NO
3
−
15
NO
3
−
10
HCO
3
−
SO
4
2
−
NO
2
−
CO
2
H
2
O
N
2
5
S(s)
CO
3
2
−
NH
3
0
NH
4
+
NH
3
−
5
NH
4
+
H
2
S
CH
4
HS
−
P
H
2
> 1 atm
−
10
45678910
45678910
45678910
4567891011
4567891011
pH
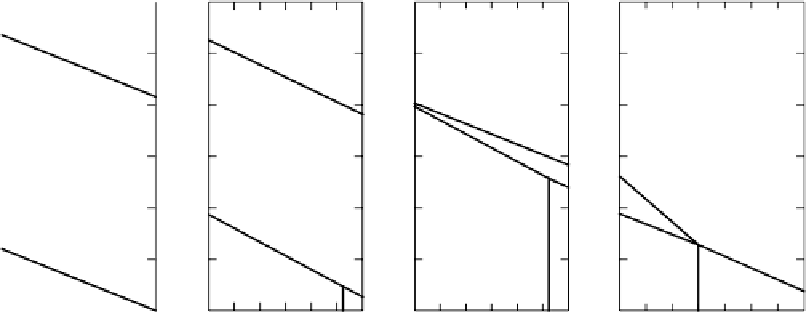
Figure 4.1
pe-pH diagrams for important biological redox couples in natural systems. (a) H
2
O-O
2
. (b) The nitrogen system consider-
ing only stable equilibria: the only oxidation states involved are
(
−
III
)
, the elemental state and
(
+
V
)
. (c) The nitrogen system treating
NH
4
+
,NH
3
,NO
3
−
and NO
2
−
as metastable with respect to N
2
which is treated as redox-inert. (d) The SO
4
2
−
-S(s)-H
2
S(aq) system,
[total soluble S]
=
10
−
2
M. (e) The carbon system ignoring elemental C. After Stumm and Morgan (1996). Reproduced by permission of
Wiley, New York