Chemistry Reference
In-Depth Information
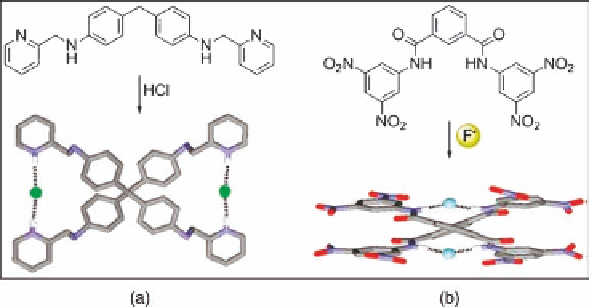
Figure 2.36 (a) Bispyridyl ligand forms an helical dimer in the presence of hydrogen chloride
in the solid state. (b) Side view of the X-ray crystal structure of the fluoride-directed assembly
of an isophthalamide cleft into a double helix.
Ref. [100]. Reproduced by permission of the
Royal Society of Chemistry.
weaker interactions with methylene and aromatic hydrogens within the binding pocket. A
similar observation was reported by Gale and coworkers for the fluoride-directed assem-
bly of an isophthalamide cleft into a double helix (Figure 2.36b) [100].
Interesting results have been obtained when the folding is driven by organic anions.
Li and coworkers reported the folding of a linear arylamide oligomer synthesized by
coupling naphthalene-2,7-diamine with 1,3,5-benzenetricarboxylic acid segments (Fig-
ure 2.37) [101]. It adopts no compact conformation without an anionic template and it
folds into a helical structure in the presence of a benzenetricarboxylate, upon hydrogen-
bonding formed with NH and CH donor groups arranged in a complementary fashion to
stabilize the complex. This complementarity towards tricarboxylate is crucial in generat-
ing the helical folded structure, as halide anions, nitrate, acetate or isophthalate do not
induce the formation of a helical complex.
Up to now, we have focused our attention to open chained oligomers, but it is possible
to program a sequence of monomers in order to obtain cyclic oligomers that display inter-
esting dynamic conformational behavior as a consequence of their size. Generally, small
cycles are relatively rigid structures with limited conformational freedom, while larger
ones are flexible and can display twisting and folding behaviors, similar to their acyclic
counterparts. The resulting secondary structures have the shape of a figure “eight,” con-
taining two binding pockets which are geometrically separated from each other.
Bohmer and coworkers presented a cyclic hexaurea (Figure 2.38), formed by four rigid
xanthenes units and two diphenyl ether units connected with six urea moieties, displaying
their NH group in the inner part of the cycle [102]. In the presence of chloride, the mole-
cule folds into two binding cavities, due to the large flexibility induced by the ether units.
Sessler and coworkers synthesized a cyclic decapyrrole named turcasarin, which can
twist to adopt a left-/right-handed enantiomeric figure of eight loops [103]. The twist
observed in the crystal structure affords two binding areas, each of which binds two chlo-
ride ions hydrogen bonded to the pyrrole NH hydrogens. With this molecule it is possible
to state that conjugated macrocycles are not necessarily rigid and flat structures, but can
Search WWH ::

Custom Search