Chemistry Reference
In-Depth Information
F
F
B
O
O
H
N
N
H
H
H
N
H
N
Figure 2.32 Dipyrrolyl diketone boron complexes are able to bind chloride, acetate and
dihydrogenophosphate ions.
Further evidence for the chelating attitude of pyrrole moieties was reported by Maeda
and coworkers, where the pyrrolic arms of the oligomer (Figure 2.32) are linked to a
diketo-substituted boron complex [91]. This particular bridge offers a modular way of
controlling the electronic and subsequent binding properties of the oligomer, which is
able to bind chloride, acetate and dihydrogenophosphate ions.
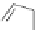
In 2005, Jeong and coworkers reported a series of oligoindoles in which monomeric
units were connected sequentially by ethynyl linkers [92]. For instance the oligomer
reported in Figure 2.33 adopts an expanded conformation in the absence of an anion, but
folds into helical conformations in the presence of a chloride, thus encapsulating the
anion within a helical conformation. The same group synthesized indolocarbazole oligo-
mers that possessed extended
-surfaces relative to the corresponding biindole-based
ones, thus possibly providing increased
p
stacking and hydrophobic interaction [93].
1,4-Disubstituted 1,2,3-triazoles are universal ligation tools [94] whose capacity for
independent function has received far less attention. Recent reports, however, indicated
that the size and dipole moment (around 5 D) of triazoles make them interesting candi-
dates for amide bond surrogates, and Arora and coworkers reported the contributions of
triazoles to the conformational preferences of peptidotriazole oligomers [95]. Craig and
coworkers reported acyclic oligomers based on the aryl 1,2,3-triazole unit [96]. The
p
-
p
=
OR
1
H
H
N
R
R
R
(
n
= 0, 1, 2)
H
N
N
R
CO
2
R
1
H
H
H
N
H
H
R
N
N
(
n
= 2)
N
H
O
CH
3
R
R
R
n
n
N
H
Ph
(
n
= 1)
Figure 2.33 Oligoindoles that can fold around an anion.
















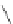












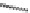

















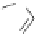







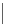


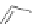

























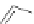

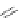










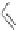
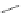












































Search WWH ::

Custom Search