Chemistry Reference
In-Depth Information
Figure 2.30 Encapsulation of an egg-shaped guest by partial unfolding of a helix possessing a
reduced diameter at both ends.
Reprinted with permission from Ref. [83a]. Copyright 2005
WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
2.4.2 Organization Induced by Anions
Anions, because of their biological and environmental relevance, are at the base of many
scientific programs within the field of supramolecular chemistry [85]. The design of syn-
thetic anion receptors with high affinity and selectivity and with a specific shape that are
capable of differentiating between anions of different size and geometry could be done
thanks to the directionality of the hydrogen bond [86]. Several foldamers exhibit strong
tendencies to form well ordered secondary structures by encapsulating guests in a non-
covalent way, within their internal cavities. As the programmed sequence of monomers
affords foldamers that fold into well defined secondary structures, it is possible to achieve
a certain secondary structure by designing the monomer. For instance, to obtain foldamer-
based anion receptors, the monomer must have hydrogen bond donors in order to interact
with anions. Some of the most used monomers contain the NH protons of amides, ureas,
pyrroles and indoles and the polarized CH protons of neutral or positively charged aro-
matic heterocycles, such as 1,2,3-triazole. The NH proton present in the pyrrole hetero-
cycle forms a strong hydrogen bond with anions [87]. Sessler and coworkers studied
several oligopeptides containing pyrrolic moieties. The exapyrrole shown in Figure 2.31
is fully conjugated, and several experiments in the solid state and in solution show that the
dihydrochloride salt exists in a planar,
S
-shaped conformation, where the two clefts coor-
dinate a chloride anion due to the formation of NH . . . Cl
hydrogen bonds [88]. In a
similar way, the arms of the di- and tri-pyrrolic moieties directly linked to a chromophore
can adjust themselves to create a concave pocket that comprises four or six NH donor
groups in a convenient manner suitable for binding an anion [89,90].
NN
NN
Cl
-
N
H
N
N
H H
N
N
N
N
H H
H
H
H
H
H
H
N
N
H
H
Cl
-
N
H
N
N
H
H
N
N
N
N
Figure 2.31 Proposed anion binding modes for receptor.







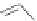

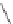



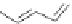

























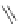




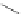
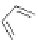
























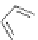




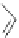





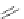






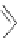

























Search WWH ::

Custom Search