Chemistry Reference
In-Depth Information
The virtues of alternating longer sequences of a-andb-amino acids, so-called
a/b-peptide foldamers, were recognized only a few years ago. During this course, several
new helix types were also discovered. In the year 2004, two groups independently investi-
gated the solution properties of a/b-peptides with a 1 : 1 backbone alternation [47].
Beside helical structures with hydrogen bonds pointing only into one direction, either
backward or forward along the sequence, also mixed helices (b-helices) with hydrogen
bonds alternately changing in forward and backward direction were found in these regular
hybrid peptides [48]. For instance, the oligomers described by the Gellman's group
revealed a “split personality,” meaning that there was a coexistence of an 11-helix along
with a 14/15-helix, rapidly interconverting in solution. For relatively long foldamers, the
14/15-helix is favored over the 11-helix [49], analogous to the a-helix that is favored over
the 3
10
-helix in larger a-peptide structures (Figure 2.12) [50].
Recently, Sharma et Hofmann [51] presented the concept of hybrid helices by the com-
bination of two or more types of homologous peptides with structural and conformational
diversity, resulting in well defined helical patterns. Sugar derived b- and g-amino acids in
combination with regular a-amino acids were used as building blocks for their approach.
Within this work, they connected b-, a/b- and a/g-peptide sequences within one oligo-
mer, combining the special features of a b-12/10-helix with an a/b-11/9-helix and
another a/g-12/10-helix (Figure 2.13).
b/g-Peptides have only recently emerged in the literature [52]. These oligomers are
nevertheless of particular interest because the backbone of a b/g-dipeptide possesses the
same number of atoms as an a-tripeptide.
Hofmann performed calculations on unsubstituted hybrid b/g-peptides [53]. He
showed that the most stable conformations were the 11- or 13-helix conformation and the
O
N
O
N
O
(a)
11-helix
H
H
H
H
O
O
O
O
N
O
N
O
(b)
14/15-helix
H
H
H
H
O
O
O
O
N
O
N
O
N
(c)
18-helix
H
H
H
H
O
O
O
Figure 2.12 Hydrogen-bond patterns that define the helical secondary structures of the
a
/
b-peptides considered here, with hydrogen bonds from carbonyl groups to amide protons in
the C-terminal direction: (a) 11-helix, (b) 14/ 15-helix, (c) 18-helix.
































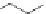



















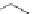






































Search WWH ::

Custom Search