Chemistry Reference
In-Depth Information
8-Helix
10-Helix
12-Helix
12
10
8
N
N
N
N
N
N
N
O
O
O
O
O
O
O
14-Helix
10/12-Helix
14
10
10
12
R
R
N
N
N
N
N
N
N
O
O
O
O
R
O
O
R
O
Figure 2.9 Nomenclature for b-peptide helices based on hydrogen-bonding patterns.
2.2.3
g
-Peptides
After three decades of studies on homogeneous oligomers containing b-amino acids, mol-
ecules based on g-amino acids were investigated. Very recently a review was published,
reporting all the work that has been done in the past 10 years on g-peptides [40].
Although this homologation reduces the number of potential hydrogen bonds for an
oligomer of the same length, the g-peptides have shown their capability to adopt various
stable conformations, such as helices, sheets and turns. In 1998, Seebach and Hannessian
reported simultaneously that homogeneous oligomers containing monosubstituted
g-amino acids can form stable helical conformations in solution [41].
Hofmann later performed calculations on unsubstituted and monosubstituted
g-peptides (with one methyl group on the a-, b-org-position), employing
ab initio
MO
theory at various levels of approximation [42]. He showed that the observed 14- and
9-helix were the most stable conformations. He also claimed that, for unsubstituted and
monosubstituted g-peptides, mixed helices could also be observed. In these cases, the
most stable helices are the 22/24- and the 14/12-helices. The hydrogen bonds are orien-
tated alternately in opposite directions leading to a small helix dipole (Figure 2.10). These
mixed helices should then be favored in less polar media. It is worth mentioning that
g
4
-peptide helices have the same screw sense and macrodipole as a-peptide helices,
whereas b
3
-peptide helices have the opposite.
Several examples have been reported recently and they confirm what was predicted by
Hoffman. All the different types of secondary structures have been observed, ranging
from helices, to sheets, turns and extended structures. For instance a 9-helix has been
observed in monosubstituted g-peptides [43]. Disubstituted g
2,4
-amino acids have also
been used for g-peptide elaboration. This additional substitution reduces the number of
accessible conformations for the backbone. In fact, only two of the nine possible con-
formers for a g-residue do not possess unfavorable
syn
-pentane interactions. Thus, Hanes-
sian synthesized tetramers and hexamers and concluded that they all adopt a right-handed
14-helix conformation in pyridine-
d
5
[44].


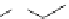
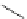



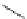

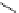
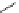

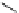




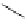

























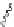


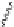

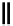
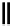
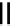





Search WWH ::

Custom Search