Chemistry Reference
In-Depth Information
O
COOH
(a) ClSO
2
NCO
aq. HCl
NH
NH
2
HCl
(b) hydrolysis
β
-Lactam route to
β
-amino acids
O
COOH
O
lipase
+
n
n
NH
n
NH
NH
2
Lipase-catalyzed ring opening of cycloalkane-fused
β
-lactam
COOMe
COOMe
COOH
COOH
CON
3
NH
2
COOMe
COOH
NH
2
COO
t
Bu
COO
t
Bu
COOH
Desymmetrization followed by Curtius degradation
COOEt
COOEt
Ru-catalysts
NHCOMe
NHCOMe
Ruthenium-catalysed enantioselective hydrogenation
Figure 2.6
Synthetic methods for the synthesis of enantiomerically pure cyclic b-amino
acids.
(MeOH, H
2
O) with chain lengths as short as four residues and without restricted
backbone rotation, as in oligomers containing 2-amino-cyclopentane- and 2-amino-
cyclohexane-carboxylic acid moieties [34].
The conformations of b-peptides can be analyzed in terms of the main chain torsional
angles, which are assigned the angles v, w, u and c (Figure2.7)intheconventionof
Balaram [35].
Folded helical or turnlike conformations of b-peptides require a gauche conformation
about the u torsion angle defined by the C2-C3 bond. A
trans
rotamer leads to a fully
extended conformation, provided the values of w and c are appropriate. The effects of
substituents on the local conformation of a b-amino acid are summarized in Figure 2.8.
b-Alanine is highly flexible, analogous to Gly in the a-amino acids. Alkyl substituents
at positions 2 and 3 favor a gauche conformation about
the C2-C3 bond [36].







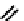

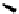














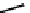





























































Search WWH ::

Custom Search